Understanding the association between persistent cervical infection with high-risk human papillomavirus (HPV) and the development of cervical cancer and its immediate precursor lesion, cervical intraepithelial neoplasia grade 3 (CIN3), is fundamental when underwriting cases where HPV is present.
The addition of HPV testing to cytology-based cervical screening has significantly increased the detection of prevalent CIN3, with a concurrent decrease in CIN3+ or cancer detected in subsequent/follow-up screening. Across studies, a positive HPV test is the strongest independent predictor of recurrent disease or progression to invasive cancer. As such, underwriting guidelines that consider HPV test findings are critical for prudent underwriting decision-making when assessing cervical screening results. Current clinical cervical screening guidelines, however, should not be changed on the basis of HPV vaccination status due to a lack of empirical data concerning the age when screening is to be initiated or the screening interval for women who have been vaccinated.
Introduction
Cervical cancer is the fourth most common cancer among women and second most common female-specific cancer, with human papillomavirus virus (HPV) genotypes 16 and 18 accounting for more than 70% of the world’s cervical cancer cases.1,2 Over the past 50 years, cytology-based screening (Papanicolaou or Pap test or smear) has been used routinely to screen women for precancerous or cancer cells in the cervix. Pap tests can detect cervical cancer and precancerous cells in the early stages, and the screening process has proven to be highly effective in reducing the number of deaths from squamous cell cervical cancer, which makes up 80% to 90% of cervical cancers3-5.
While the Pap test can detect abnormal cell changes, testing for HPV detects the viral infection that can cause the abnormal cell changes prior to the development of cancer. Importantly, numerous studies have shown that screening for HPV leads to earlier detection of cervical intraepithelial neoplasia (CIN) than cytology. Accordingly, the U.K., Australia and several countries in Europe have introduced HPV primary screening into their national screening programs6. This article discusses the important role HPV testing plays in cervical cancer screening, changes to the screening guidelines with HPV as a primary screening tool, and its direct impact on the way Pap smear results are currently underwritten in an insurance setting.
Human Papillomavirus
Studies have confirmed that persistent cervical infection with high-risk human papillomavirus (HPV) is necessary for the development of cervical cancers (both squamous and adenocarcinoma) and its immediate precursor lesion, cervical intraepithelial neoplasia grade 3 (CIN3)7,8.
Currently, more than 100 HPV genotypes have been identified9. The two high-risk HPV genotypes responsible for about 70% of all cases of cervical carcinomas are HPV 16 and HPV 1810,11,12,13, with one study observing 74% of squamous cell carcinomas and 78% of adenocarcinomas testing positive for either type14. HPV 16 accounts for approximately 55% to 60% of all cervical cancers while HPV 18 accounts for approximately 10% to 15%. Approximately 10 other HPV genotypes cause the remaining 25% to 35% of cervical cancers7,8,15.
The majority (~90%) of HPV infections are transient and become undetectable within one or two years16,17. Infections which are persistent have a high risk of developing into precancerous lesions. For example, a persistent two-year infection of HPV 16 strongly predicts CIN3 or a more severe diagnosis (CIN3+) in the following years (i.e., 20% to 30% risk of CIN3+ over five years for one-year or two-year persistent HPV 16). If left untreated, CIN3 has a 30% probability of becoming invasive cancer over a 30-year period. However, if treated, only approximately 1% will become invasive18.
Updated Terminology
There are multiple nomenclatures for reporting HPV-associated lesions. Starting in the mid-1960s, premalignant squamous changes of the cervix were classified as mild, moderate, or severe cervical dysplasia. Dysplasia is a lesion in which part of the thickness of the epithelium is replaced by cells showing varying degrees of atypia (i.e., structural abnormalities).
In 1988 the Bethesda system was introduced. In this system, which underwent revisions in 1991 and 2001, different terminology was used for cytologic (Pap test) and histologic (biopsy) findings. Cytologic findings were described with the term “squamous intraepithelial lesion (SIL)” whereas histologic findings were described with the term “cervical intraepithelial neoplasia (CIN)”19. The term “cervical intraepithelial neoplasia” (CIN) was originally introduced by Richart to present the concept of cervical neoplasia as a disease continuum20.
In 2012, the Lower Anogenital Squamous Terminology (LAST) consensus project was convened to reassess and harmonize the biopsy and cytology terminology used to report HPV-associated squamous lesions of the lower anogenital tract. The LAST nomenclature, which is now the global standard, relies on HPV 16 staining (though not routinely recommended) to triage CIN2. CIN2 with HPV 16-positive is classified as CIN3, representing a high-grade squamous intraepithelial lesion (HSIL), the immediate precursor to cervical cancer. In contrast, CIN2 with HPV 16-negative is classified as CIN1, representing a low-grade squamous intraepithelial lesion (LSIL), the histologic sign of HPV infection21,22.
Table 1
Histology and Cytology Terminology
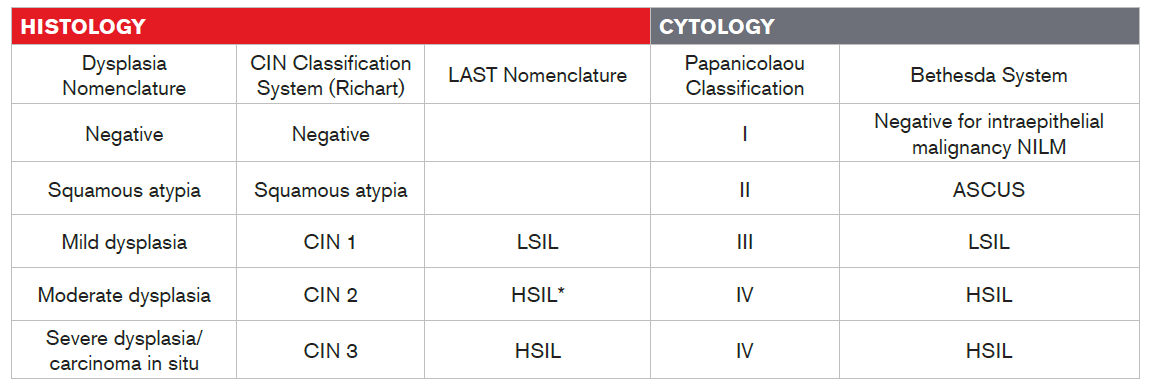
* Per LAST nomenclature, CIN2 with HPV 16-negative test is considered as LSIL
Recurrence, Follow-up, and HPV Co-Testing
A long-term multi-cohort study observed that the five-year risk of post-treatment CIN2 or higher in women with three consecutive negative cytology or negative co-testing (Pap smear and HPV test) results at six and 24 months was similar to that of women with normal cytology results in population-based screening. This justifies returning post-treatment women to regular screening schedules23. HPV co-testing also has the benefit of diagnosing cervical adenocarcinoma and adenocarcinoma in situ, which traditional cytology often fails to detect24.
The Katki et al. study of five-year risk of CIN2+ for three follow-up testing strategies after treatment (Pap test alone, HPV alone, and co-testing) indicated that women with prior diagnoses of atypical glandular cells of undetermined significance (AGC), atypical squamous cells – high-grade lesion (ASC-H), or high-grade intraepithelial lesion (HSIL+) Pap test results treated for CIN2 or CIN3/adenocarcinoma in situ (AIS), had a substantial risk of developing CIN2+ post-treatment. However, post-treatment risk was dependent on the severity of the treated histology (CIN2 = 10% versus CIN3/AIS = 16%). The authors concluded that after negative test results post-treatment, no women had achieved risk sufficiently low to permit them to return to five-year routine screenings. Furthermore, negative co-tests after treatment were associated with a lower (five-year) risk of recurrent CIN2+ (one test = 2.4%; two tests = 1.5%) than either a negative HPV test (one test = 3.7%; two tests = 2.7%) or Pap test(s) alone (one test = 4.2%; two tests = 2.7%), although the differences were not significant due to considerable uncertainty around estimates because of the relatively small numbers of recurrences observed25.
A retrospective analysis of long-term clinical outcomes after treatment for high-grade cervical lesions found that progressive disease was detected only in the first year after treatment. The main predictors of long-term outcomes depended upon the type of transformation zone, the lesion grade, the status of the margins and the result of HPV testing at six to 12 months follow-up26. The study by Costa et al. of the performance of HPV DNA testing in the follow-up after treatment of high-grade cervical lesions, adenocarcinoma in situ and micro-invasive carcinoma observed that HPV testing was significantly more sensitive (95%) compared to follow-up cytology (70%) in detecting post-treatment squamous intraepithelial high-grade lesions. Furthermore, for individuals who had been treated conservatively for cervical adenocarcinoma in situ, HPV testing was the strongest independent predictor of recurrent disease or progression to invasive cancer, and co-testing achieved 90% sensitivity in detecting persistent lesions at the first follow-up visit and 100% at the second follow-up visit27.
A study of long-term risk of invasive cervical cancer after treatment of squamous cell intraepithelial neoplasia found that after the first year following treatment for CIN, the rate of invasive disease remained about 56 per 100,000 woman-years until at least 20 years after treatment. In contrast, the risk of post-treatment CIN declined steadily with time to about 190 per 100,000 women in year 10. Although the post-treatment rate of CIN falls with time, the rate of invasive disease remains static. Therefore, annual Pap smears are recommended for at least 10 years post-treatment28. Guidelines for the U.K.’s National Health Service (NHS) Cervical Screening Program, for example, recommend women have annual follow-up tests for at least 10 years after the treatment of CIN2 or worse before returning to the routine screening interval. Women treated for CIN1 can be returned to routine recall (i.e., screening) after two years of negative post-treatment cytology tests29.
Medical Guidelines for Screening
In cervical cancer screening, the addition of HPV testing to cytology testing has increased the detection of prevalent CIN3 and a simultaneous decrease in the detection of CIN3+ or cancer in subsequent and follow-up screenings30,31,32, yielding a decrease in the development of invasive cancers such as SCC (squamous cell cancers) and invasive adenocarcinoma of the cervix. Cytology testing alone, however, has been relatively ineffective in reducing the incidence of invasive adenocarcinoma of the cervix33 34. A systematic review of randomized studies of HPV testing conducted for the U.S. Preventive Services Task Force showed that HPV screening was consistently more sensitive than Pap tests for the detection of ≥CIN2 and ≥CIN335.
In addition, a number of studies have reported that co-testing detected a greater proportion of CIN3+ in the first round of screening compared to cytology alone30,31,32. Research completed by the International Collaboration of Epidemiological Studies of Cervical Cancer Group pooled screening data from 12 epidemiological studies involving 1,374 women with adenocarcinoma and concluded that the reduction of risk due to a preceding cytologytest was greater for squamous cell carcinoma than for adenocarcinoma11. Testing positive for HPV is strongly associated with a diagnosis of cervical adenocarcinoma36.
The latest cervical cancer testing guidelines, published in the Journal of Global Oncology, recommend that asymptomatic women in all settings (i.e., medical circumstances) undergo HPV DNA testing. Table 2, below, outlines the recommendations37.
SETTING
|
AGE
| SCREENING
FREQUENCY |
|---|
| Maximal source setting:May use high-resource setting guidelines; high level/state-of the art resources or services that may be used in some high-resource countries and/or may be recommended by high-resource setting guidelines that do not adapt to resource constraints. This should be considered lower priority than in the other settings on the basis of cost impracticality for limited-resource environment. | 25-65 | Screen every five years. |
| Enhanced setting: Third-tier resources or services that are optional but important. Enhanced-level resources may produce further improvements in outcome but increase the number and quality of screening/treatment options and individual choice (perhaps ability to track patients and links to registries). | 30-65 | Women in this age range who have had two consecutive negative HPV tests five years apart can extend their screening intervals to every 10 years. |
| Source limited setting:Second-tier resources or services that produce major improvements in outcomes, such as incidence and cost effectiveness, but that are attainable with limited financial means and modest infrastructure; limited-level services may involve single or multiple interactions; universal public health interventions are feasible for a greater percentage of the population than primary target group. | 30-49 | Women in this age range can be screened every 10 years. |
| Basic setting:Core resources or fundamental services absolutely necessary for any public health/primary health care system to function; basic-level services typically are applied in a single clinical interaction; screening is feasible for highest need populations. | 30-49 | Women in this age bracket may be screened one to three times in their lifetimes. |
In Europe, cervical cancer screening guidelines recommend the use of HPV testing (high-risk HPV 16 and 18) to triage women and as a standalone primary screening test without cytology. Guidelines from the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists (ACOG) and the American Society
for Colposcopy and Cervical Pathology (ASCCP) suggest that cervical cancer screening should only begin at age 21, as evidence shows that cervical cancer is rare among adolescents and young women38. Australia’s National Cervical Screening Program recently announced that its current screening program will be renewed in May 2017 and will invite women aged 25 to 74 years, both HPV vaccinated and unvaccinated, to undertake an HPV test every five years.
In July 2016, it was announced that primary testing for HPV of cervical screening samples would be rolled out across all of England. Cervical screening guidelines for the country are recommending that routine HPV and Pap screenings be offered to women as follows:
- Ages 25 to 49 – every three years
- Ages 50 to 64 – every five years
- Age 65 and older – only to women who have not been screened since age 50 or who have recently had abnormal tests
All of the aforementioned guidelines include women who have had the HPV vaccination, as the vaccine doesn’t guarantee complete protection against cervical cancer39.
HPV Vaccination and Screening
Two HPV vaccines have been licensed globally since 2006. The first was Cervarix®, a bivalent vaccine that targets HPV 16 and 18. The second was Gardasil®, which additionally targets HPV 6 and 11 (which are responsible for about 90% of anogenital warts)40. In the U.S., one systematic review and meta-analysis of research found that HPV vaccine (HPVV) uptake varied by ethnicity and healthcare coverage41. In addition, although the HPVV is provided free of charge in most European countries, a recent systematic review also found that ethnocultural and educational factors play an important role in HPVV uptake in Europe42.
Both the European guidelines for quality assurance in cervical cancer screening and the ASCCP have recommended that current cervical screening practices should not be changed on the basis of HPV vaccination status at present5,43. This is due to a host of factors, including: a lack of empirical data concerning the age when screening is to be initiated or in the screening interval for women who have been vaccinated; that vaccinated women are unprotected from 30% of cervical cancer cases (due to other HPV genotypes not included in the vaccine); that many women who have been vaccinated did so subsequent to HPV exposure (decreasing efficacy); poor vaccination compliance rates; and geographic and socioeconomic disparities in vaccination coverage both within and across countries5,37,43,44. Similar screening recommendation guidelines are given by the WHO (World Health Organization) and FIGO (International Federation of Gynecology and Obstetrics)45,46.
Conclusion
The introduction of HPV as a primary screening tool is becoming standard practice in many parts of the developed world, with developing countries most likely to follow in kind. Understanding HPV and its role in cervical carcinogenesis is crucial when assessing HPV test and cytology results. Having appropriate underwriting guidelines which cater to HPV test findings is essential if cervical screening results are to be used accurately and consistently when making underwriting decisions.