Within the last few years the term “liquid biopsy” has come into common usage and the concept is receiving significant attention in both the medical and non-medical literature.
Great hope (and perhaps hype) have also been generated, with claims of liquid biopsies becoming a game-changer primarily in the diagnosis and treatment of cancer. Driving this forward is significant large-scale investment5 in this new and rapidly developing technology, both commercially and academically.
This paper will describe the current status of liquid biopsies in the context of cancer, discuss their multiple potential applications, and assess the possible impact on mortality and morbidity. Specific opportunities and challenges they may bring to the insurance industry will also be reviewed, and RGA’s perspectives presented.
Definition of Liquid Biopsy
A liquid biopsy is any type of test which looks for signs (genetic material, biomarkers, or cells) of cancer in a person’s bodily fluids – most often in blood (the primary focus of this paper), but also in urine, saliva, semen or other fluids14
Standard tissue biopsy – that is, of tumor tissue – is currently the gold standard for cancer diagnosis and for clinical and molecular profiling of tumor DNA. Tissue biopsies, however, have a number of disadvantages, including tissue inaccessibility, cost, invasiveness, chance of inadequate sampling, and some pain and risk to the patient.
Liquid biopsies have several advantages: they are easily obtained, minimally invasive, quick, and incur minimal patient pain and risk.2 However, a significant current limitation of liquid biopsies is the ability to clearly differentiate genetic variations which may be present in healthy individuals compared to those with cancer.6 Furthermore, genetic variants or mutations need to be identified as “drivers” (i.e., actively driving the cancer pathogenesis) vs. “passenger” (i.e., present, but of no known pathological significance) in order to understand the clinical significance.
Additionally, discussion has also centered on variations in the capability of liquid biopsies to determine cancer tissue of origin or topographic location. While confidence remains high regarding the success of liquid biopsies, there have been some setbacks in the developmental and research process. While these are not unexpected with any new or emerging technology, the general expectation is that over time there will be great technical success.
Types of Liquid Biopsy Tests
Circulating cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA)
Circulating cell-free DNA (cfDNA) are cell-free fragments of DNA that are routinely shed into the circulatory system by all cells in the body. Circulating tumor DNA (ctDNA) is cfDNA that is shed specifically by tumor cells and contains the same genetic variants as those in a primary or metastatic tumor.
ctDNA enters the bloodstream through a variety of mechanisms: via secretions from viable tumor cells as either cfDNA or in cell-derived vesicles known as exosomes; via secretions from phagocytes (a type of immune system cell) post-engulfment of tumor cells; or as a result of tumor cell death through necrosis and apoptosis (programmed cell death).2
This type of liquid biopsy is a non-invasive method of extracting ctDNA from blood samples for the detection and analysis of tumor DNA. Liquid biopsies of ctDNA also allow for the detection of somatic genetic mutations that are present only in the DNA of pre-cancerous or cancerous cells and not in normal cells. As such, ctDNA serves as a very specific biomarker.
Advances in digital genomic technologies have helped overcome the fact that ctDNA often makes up <1% of the total cfDNA in individuals with solid malignancies. These advances mean liquid biopsy is now highly sensitive and allow for mutation(s) in the tumor tissue to match the mutation(s) found in the ctDNA.1
One of the most significant limitations of a standard tissue biopsy is that most advanced cancers are heterogeneous. This means that aside from interpatient tumor heterogeneity (e.g., the same type of tumor can vary between two different people), there can also be intratumoral heterogeneity, where different genetic profiles are found within the same person’s tumor. In addition, there can be intrametastatic heterogeneity, where varying genetic profiles can be found among metastases in the same person. A standard tissue biopsy from just one portion of the tumor or one metastasis may miss this molecular complexity.
Since liquid biopsy of ctDNA contains the same variants as those in all the tumor cells throughout the body, it is assumed that ctDNA is released from diverse tumor sites and heterogeneous tumor cell types. Thus a liquid biopsy should allow for the capturing of tumor and metastatic heterogeneity and the assessment of cancer in real time.1
Additionally, fragments of cell-free nucleic acids (cfNA) such as cfDNA and ctDNA, and circulating non-coding RNAs (ncRNAs) such as messenger RNA (mRNA), micro RNA (miRNA) or long ncRNA (IncRNA),3 can be detected not only in plasma or serum but also in urine, cerebrospinal fluid, or even ascites of patients with cancer.4 Given that this area of research is expanding beyond the measurement of only ctDNA in blood, some experts are of the opinion that the term “liquid biopsy” may not be adequate and that this type of testing might be more appropriately referred to as, for example, “ctDNA in plasma” or “miRNA in urine,” thus providing a more specific and comprehensive description of the test.
Circulating tumor cells (CTCs)
This type of liquid biopsy detects the presence of whole tumor cells in the bloodstream, which are known as circulating tumor cells (CTCs). These cells, first described in 1869, were thought (correctly, as it turns out) to be related to the development of metastases.
Preliminary data suggest that CTCs may also have a role in early cancer detection in a high-risk group as they may be present in the blood even before someone has cancer symptoms or clinically detectable cancer. According to a study which looked at whether CTCs could signal the presence of lung cancer in people with chronic obstructive pulmonary disease (COPD), CTCs may have value for early cancer detection in this high-risk group. Researchers found CTCs in 3% of the participants with COPD but no detectable cancer. However, each of these patients, who were monitored closely, developed lung cancer between one and four years later. Another important aspect of this research is that none of the participants without detectable CTCs developed lung cancer up to five years after the study started.14
Additionally, one of the strengths of CTC analysis is that it not only allows pure (and complete) tumor DNA to be assessed but pure tumor RNA as well,6 thus, allowing for tumor transcriptome analysis.
Emerging Technologies
The field of liquid biopsy continues to develop, and newer techniques are being pioneered. We should therefore anticipate further publications and discoveries with regard to the use of epigenetics, plasma RNA analysis, novel plasma DNA preparation protocols, and novel exosome technologies. Significant research is also focusing on host autoantibody and other immune system responses to cancer and neoantigens associated with cancer, which could be leveraged as screening and diagnostic markers of cancer.
Potential Clinical Uses
As with any clinical test, liquid biopsy must be evaluated by the following strict criteria: analytical validity, clinical validity, and clinical utility. As this field’s technology is advancing rapidly, close attention must be paid to ensure these criteria are met for both clinical and insurance medicine purposes.
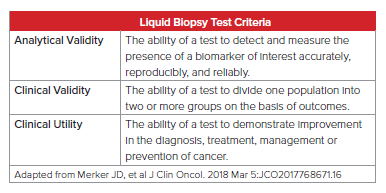
According to Daniel Hayes, M.D., of the University of Michigan Comprehensive Care Center and former president of the American Society of Clinical Oncologists, “the bottom line is clinical utility – does the result of the assay lead to a clinical decision that’s been shown with a high level of evidence to improve outcome?” He goes on to indicate that doctors need to determine clinical utility before this testing can be applied to patients.10
Furthermore, Lynn Sorbara, Ph.D., of the National Cancer Institute (U.S.) recently stated, “I think the major stumbling block for moving these liquid biopsy tests forward is there is not enough clinical verification and validation to know and feel comfortable that what we’re detecting with them is clinically meaningful.”11
Keeping the above test criteria in mind, along with current medical opinion, the potential uses of liquid biopsy are frequently grouped into the following categories with regard to cancer:
- screening
- treatment selection and real-time monitoring of response (prognosis)
- identification of resistance mechanisms
- detection of recurrence
Undoubtedly, the greatest hope of liquid biopsy is its potential ability to revolutionize early detection of and screening for cancers. It is generally believed that curative therapies are most successful when cancer is diagnosed and treated at an early stage. While this paradigm may generally be true, screening can identify potential tumors that may have no impact on life expectancy and may expose the individual to unnecessary testing and potentially higher morbidity.
Statistically, earlier detection of a cancer without actual improvement in survival is referred to as “lead time bias” and must be controlled for in clinical studies to avoid attributing erroneous value to the screening test.
While research continues aggressively in the cancer screening space, the biological, technical, and clinical obstacles to developing a safe and effective pan-cancer screening test are significant.12
FDA-Approved Tests
Circulating tumor DNA (ctDNA) test
The Food and Drug Administration (FDA) (U.S.) approved the cobas® EGFR Mutation Test v2 in 2016 for ctDNA. This liquid biopsy test, currently the only one approved by the FDA for ctDNA testing, is used in non-small-cell lung cancer (NSCLC) to look for mutations in one specific gene – EGFR –and indicates whether drugs that target these mutations, such as erlotinib and osimeritinib, could work against the cancer. This test has the added advantage of providing molecular information about the cancer, so that patients can be identified who can benefit from drugs that target these mutations, and treatment can be guided by tracking patient responses to therapy.
This test, however, has limitations, as it may produce a false negative result. The FDA recommends a tissue biopsy if the liquid biopsy is negative (i.e., if it does not detect an EGFR mutation). There are other commercially available liquid biopsy tests for other solid tumors, but they have not yet received FDA approval.11
Circulating tumor cells (CTC) test
Even though many CTC tests are commercially available, the FDA has approved only CELLSEARCH®, which measures CTCs and offers prognostic evaluation in patients with advanced breast, colorectal, and prostate cancers. By counting CTCs at different points of time (e.g., before and after initiating therapy), physicians can assess tumor response and both progression-free and overall survival.
Researchers have also been moving towards analyzing detected CTC content (e.g., miRNAs) as possible cancer biomarkers. These tests show remarkable potential, especially in prognostic evaluation in urological malignancies.13, 14
Insurance Implications
Potential clinical applications of liquid biopsy are incredibly exciting and, given their multiple potential uses, will surely impact many aspects of insurance medicine and products. Despite technical challenges and the need for further clinical validation, there is great hope for breakthrough developments in liquid biopsy technology and precision medicine, leading to overall reduction of cancer-related mortality and morbidity. Thus, while currently presenting some challenges to the insurance industry, liquid biopsy technology also represents great opportunity. Additionally, given the fact that many types of liquid biopsies are genetically based assays, appropriate use of these test results by the insurance industry will need to be assessed carefully due to various genetic regulatory restrictions, which vary by jurisdiction.>
Life insurance
With real-time monitoring and targeted therapies guided by liquid biopsy even uniformly fatal cancers, such as advanced-stage lung cancers and melanomas, can be controlled for extended periods of time with an array of relatively well-tolerated therapies that, to date, exhibit very modest long-term risks. Nonetheless, longer-term survival will need to be demonstrated before there will be a significant impact on the underwriting of affected individuals and on mortality outcomes for insured lives. Eventually, post-issue or in-force wellness management programs may be considered once improved outcomes are proven.
Critical illness (CI)/cancer cover
If liquid biopsies are eventually used as viable pan-cancer screening tests, the potential for over-diagnosis may rise and an increase in cancer incidence rates may result. This could have a significant impact on in-force products with guaranteed pricing and could also change how new products are priced. Added to this could be the risk of anti-selection at underwriting, leading to more applicants with positive screening tests selectively purchasing critical illness or cancer covers.
As more than half of CI claims are due to cancer, any advances in cancer diagnosis capabilities may significantly impact this benefit. Currently, well-designed cancer definitions for CI products require histopathology or tissue biopsy reports. The impact of liquid biopsy on standard, staged, and severity-based CI products remains to be determined. The design of these products is often based upon traditional histopathological and topographical staging. However, if liquid biopsy challenges the classic taxonomy of tumor staging and progression, unfavorable claims experience may result.
There has been speculation about whether standard tissue biopsy may someday be replaced by liquid biopsy. Experts agree that despite the various advantages of liquid biopsy tests, ctDNA testing is not intended to replace traditional tumor biopsy testing entirely but will have its own place. A recent study of NSCLC patients did suggest that ctDNA testing is a viable choice when there is insufficient tissue for genomic testing or repeat tumor biopsy is not possible.7 Having said that, all these cases would also likely have other companion investigations, such as imaging, to stage the cancer and the final diagnosis will not be based on liquid biopsy in isolation. In addition, clinical treatment with radiotherapy, chemotherapy, or immunotherapy would significantly support the cancer diagnosis and hence may be covered per the level of severity intent of the CI policy.
Terminal Illness
With improved overall survival and progression-free survival rates for those with cancer, terminal illness cover may lose some of its attractiveness given that its policy terms of death within one (or otherwise specified) year may no longer be fulfilled. Assessing life expectancy of those with advanced cancer is difficult enough, thus the introduction of liquid biopsy and precision treatment would introduce more variability and potentially make that process even more complicated.
Health and medical reimbursement
As more biotechnology companies develop liquid biopsy tests, the costs of performing such tests over time could drop to the point that they might be more cost-effective than standard tissue biopsies. The average cost of tissue biopsy in lung cancer can be as high as $15,000.8 The cost is much higher in the event of any complications requiring hospitalization. The liquid biopsy test GeneStrat®, for example, is significantly less expensive than standard tissue-based biopsies, with an average savings of approximately $3,300 and $7,400 for CT-guided and navigational bronchoscopies, respectively, in patients with confirmed NSCLC.9
Presently, only a few liquid biopsy tests are included in standard clinical guidelines for cancer care. However, with more FDA approvals these tests will likely be included in reimbursement benefits by third-party public and private payers.
Insurance Definitions and Claims Adjudication
Historically, medical advances have benefited overall population health and wellness and have led to improved morbidity and mortality outcomes. Thus, it is likely that liquid biopsy, in some form, may be no different. However, certain advances, such as the ever-changing definition of myocardial infarction, have impacted insurers unfavorably on certain product lines such as critical illness.
Keeping this in mind, insurers should re-evaluate definitions and, in the case of liquid biopsy, strongly consider modifying cancer definitions for critical illness policies to address its aforementioned potential impact on incidence and claims rates. To that end, consideration may be given to the addition of a well-crafted liquid biopsy exclusion. However, it or any other cancer definition modification should be designed to ensure consumer fairness and transparency, to allow for changes in clinical standards, and to enable claims to be adjudicated with an objective perspective so that decisions remain reasonably consistent with clinical standards.
Summary
The rapid development of liquid biopsies along with the potential for multiple clinical applications, including new cancer screening tests, has garnered significant attention in both the medical and non-medical literature. This area of research is also receiving large sums from the investment sector with high expectations for profitable returns. Historically, medical advances which have improved mortality and morbidity have also, either directly or indirectly, benefited the insurance industry. Thus, despite the challenges and concerns regarding the emerging field of liquid biopsy, insurers should seek ways to embrace and find opportunity with these promising developments.







