Non-small-cell lung cancer (NSCLC) is one of the two main types of lung cancer, accounting for about 85% of cases.
NSCLC is a heterogeneous group of cancers that includes adenocarcinoma, squamous cell (epidermoid) carcinoma, and large cell (undifferentiated) carcinoma. Small cell lung cancers (SCLC), which represent the rest, have only two main subtypes – small cell carcinoma and combined small cell carcinoma.
Computed tomography (CT) scans, which provide three-dimensional images, can detect non-small-cell lung cancers at earlier stages than other scanning methods, allowing it to be treated with surgery. Radiation therapy improvements over the past two decades have made it a viable treatment alternative as well.
Unfortunately, the majority of those with lung cancer continue to be undiagnosed until the cancer is at an advanced stage. The American Cancer Society reports that only 16% of lung cancers are diagnosed at a localized stage – that is, while the tumor is still confined to the lung2.
Prevention of smoking and cessation of smoking offer the most important route to decreasing morbidity and mortality, as approximately 90% of cases are due to smoking. With the introduction of molecular tumor testing (or biomarker testing), which looks at tumor DNA mutations and levels of specific proteins, it is now possible to individualize systemic treatment for NSCLC. Expanded drug therapies (including targeted agents) and immunotherapy advances means that systemic treatment can now be optimized for each individual. As a result, life expectancies as well as quality of life have been improving for lung cancer patients: survival rates, according to the National Cancer Institute’s SEER database, are 18.1% for patients treated between 2007 and 2013 versus 15.7% between 1995 and 2000.
Screening for Lung Cancer
Although screenings have been proven effective in detecting earlier stage lung cancers, adoption is not yet worldwide. Screening has long been well-accepted in the U.S.: The National Lung Screening Trial3, conducted in the U.S. from 2002 to 2009, demonstrated that annual low-dose single CT (LDCT) scans reduce lung cancer mortality in high-risk individuals based on age and smoking history. Compared with chest x-rays – an older, traditional mode of screening – the relative reduction in deaths was 20% and the absolute reduction 62 per 100,000 person-years. The trial also found that the number of individuals that needed to be screened in order to prevent one lung cancer death was 320.
In the U.S., lung cancer screening was expanded in February 2015 by Medicare, which covers people age 65 and older. Smokers of at least 30 pack-years, or who are between the ages of 55 and 74 and quit less than 15 years ago, are considered good screening candidates under American Cancer Society guidelines for annual CT scans.
A key question: are lung cancer screenings cost-effective? At this point, that’s not an easy question to answer. LDCT, in both trials and practice, has been found to be associated with a false positive rate of greater than 90%. The need for repeat scans and invasive procedures for these individuals could cause physical and psychological harm.
A recent study of Veterans Health Administration4 (U.S.) efforts to set up a comprehensive lung cancer screening service highlighted both the logistical difficulties in performing screenings and the immense resources required to do so effectively.
In contrast to the National Lung Screening trial, the veterans population not only had significant comorbidity, but also incidental findings such as emphysema, other pulmonary abnormalities, and coronary artery calcification in 40.7% of the patients screened. This can complicate or confound lung cancer screening results.
Screening trials are also currently underway in Europe, but in Asia, which has 51% of lung cancer deaths worldwide, only Japan and Korea have well-established lung cancer screening programs. Other countries – most notably China – are investigating emplacing screening programs.
In Japan, where lung cancer is a leading cause of mortality for both men and women, nationwide screening started in 1987. The screens consisted of chest x-rays and sputum cytology (another way of testing who might be at risk for developing lung cancer), and annual LDCT screening was added in 1993. In Hitachi City Prefecture, where low-dose CT screening among employees and communities began in 2001, lung cancer mortality among employees and in the community of those aged 50-69, in the period from 2005 to 2009, fell by 24% compared with national statistics5.
NSCLC Diagnosis and Staging
The most common signs of lung cancer are cough, hemoptysis (blood in sputum) and dyspnea (difficulty breathing). These symptoms often represent advanced-stage lung cancer. In cases where distant metastases have occurred, common sites are liver, adrenal glands, bones, and brain.
Tumor biopsy and histology are essential for confirming a lung cancer diagnosis and for determining if it is a metastasis from a different primary cancer. Testing for molecular or genetic markers now guides systemic therapy for advanced and recurrent cancers.
Positron emission tomography-computed tomography (PET-CT) scans are more accurate for evaluating the mediastinal lymph nodes with positive predictive value of approximately 75%6. False positive results are seen with infection, inflammation and granulomatous disease. Biopsy may be required for clarification before proceeding with surgery.
Treatment of Early-Stage NSCLC
Only 30% of those diagnosed with NSCLC have Stage I or II disease. Its cure rate has improved with aggressive multi-modality treatment. Currently, the definitive treatment is lobectomy. Video-assisted thoracoscopic surgery reduces incidence of complications and duration of hospital stay without compromising outcomes. Post-operative radiotherapy is considered for tumor at resection margin or positive lymph nodes to improve locoregional control7.
Chemotherapy for Stage II tumors is associated with a 5% survival advantage8. Certain Stage I tumors greater than 4 cm and other adverse features such as poor grade, lymphovascular invasion and high uptake on PET-CT scan, may also receive chemotherapy. For Stage II and IIIA, the addition of chemotherapy to surgery achieves 33% survival at five years9.
Radical radiotherapy (also known as external beam radiation therapy, or EBRT), a non-surgical treatment involving x-rays, is done on patients who are not medically fit for surgery. For Stage I peripheral tumors, stereotactic ablative radiotherapy (SABR) where available is considered best practice.
The thorax is a difficult site to treat with radiotherapy due to the low density of the lung, breathing movements during treatments, and the proximity of critical structures in the mediastinum. Recent innovations in treatment planning and delivery have led to more precise and accurate targeting of treatment.
3D conformal radiation treatment techniques utilize CT scans to shape treatment volumes and optimize dosimetry. Sharper dose gradients can be achieved with intensity-modulated radiation therapy (IMRT), giving the option of treating larger tumors. These techniques have also reduced the rate of radiation-induced esophagitis. Whether adjusting treatment volumes during a course of radiotherapy as the tumor shrinks can be beneficial is under study.
Respiratory gating 4D techniques can reduce the risk of geographical miss, which occurs due to the up-and-down movement from breathing during treatments. To further refine the gating, MRI visualization during treatment is being explored so that radiation delivery can be switched off when the target treatment volume is outside a pre-specified safety margin10.
Stereotactic ablative radiotherapy (SABR) is able to deliver high doses of radiation to a defined target volume using multiple convergent beams, with rapid dose fall-off at the edge of the volume, which reduces dosage to adjacent normal tissue. Usually three to eight daily treatments are given, whereas conventional radiotherapy treatments are over four to seven weeks.
SABR may also be a viable alternative to surgery for operable tumors as Phase II trials indicate similar outcomes11, 12. The failure rate for regional lymph nodes is 15% and at distant sites 20% for both SABR and surgery13. However, due to risk of severe damage, it is not safe to employ SABR for central tumors within two centimeters of any critical structure in the mediastinum such as bronchial tubes, esophagus, heart, major blood vessels, nerves and spinal cord. The role of SABR in surgical patients is under study in four trials.
Treatment of Unresectable NSCLC
Stage III NSCLC remains a challenging disease to treat. Chemoradiotherapy – that is, having both chemotherapy and radiotherapy treatments together – is the international standard of care, but can only be tolerated by those with excellent performance status14. The median survival rate for those who have undergone chemoradiotherapy is 17 to 28 months, and after five years, only 20% to 30% are still alive. Rates of treatment failure (disease recurrence) are 30% to 40% in the chest and 40% to 50% at distant sites15.
If using radical radiotherapy alone for these tumors, continuous hyperfractionated accelerated radiotherapy (CHART) and shortening treatment duration to 12 days
with three equally spaced fractions (or treatments) daily has been shown to improve outcomes. Overall survival rates at two, three and five years are 29%, 20%, and 11%, respectively16.
Treatment of Stage IV NSCLC
The majority of people with NSCLC present with advanced disease, and treatment goals are controlling disease, relieving symptoms, improving life expectancy, and optimizing quality of life.
The recognition that patients with small volume disease (i.e., a tumor or lesion that can be removed surgically or treated with a moderate to high dose of radiotherapy) in a single site or multiple distant sites can achieve long-term survival has led to more aggressive treatment management, with selective use of ablative surgery and radiotherapy in addition to systemic therapy. Small studies have reported two-year survival rates of 23% to 38%, and five-year survival rates of 14%, even for those where the cancer metastasized to the brain17.
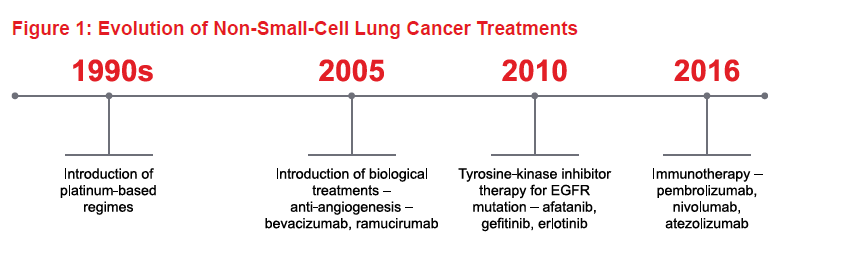
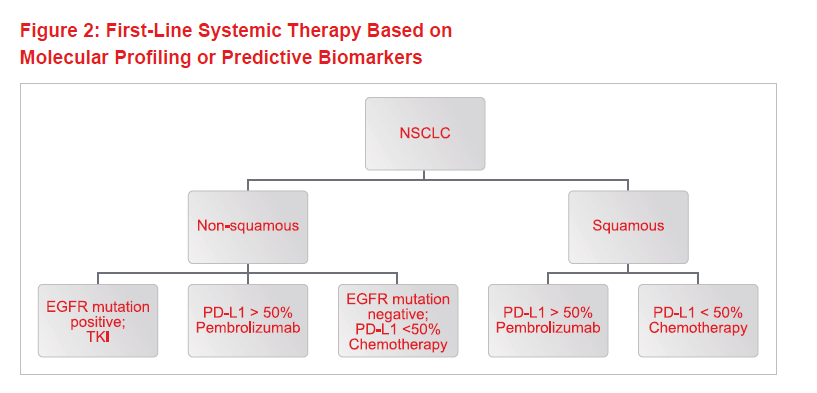
In recent years, major advances in our understanding of the molecular biology of NSCLC has led to an explosion of choices for systemic therapy. Figure 1 shows the time scale for the evolution of treatments over the past century-plus. Today, treatment is guided by histology and molecular markers. Figure 2 shows the decision-making process for first-line NSCLC therapy, based on molecular profiling or predictive biomarkers for biological treatments and immunotherapy.
For adenocarcinoma, tyrosine-kinase inhibitors (TKIs) such as erlotinib and gefitinib are used in the presence of tumors that show epidermal growth factor receptor mutation(s). For NSCLC where high levels of the protein PD-L1 are present, immunotherapy has now supplanted chemotherapy as first line treatment. Comparing such biological treatments with chemotherapy, the side-effect profile is different and often less frequent and less severe.
Immunotherapy with Checkpoint Inhibitors
NSCLC has the ability to evade attack by the immune system. About 30% of cases have high levels of PD-L1. When this ligand binds to the PD-1 checkpoint protein on T cells, the immune system is switched off. Blocking this pathway with the checkpoint inhibitor pembrolizumab allows T-cell activation.
The pivotal KEYNOTE-024 trial18 with 305 patients shows that pembrolizumab is superior to cisplatin doublet chemotherapy regimens as first-line therapy. The objective response rate was 44.8% vs 27.8%. Median progression free survival with pembrolizumab was 10.3 months, compared with six months for chemotherapy. The one-year survival was 70% vs. 54%, and the risk of death reduced by 40%.
As a result, pembrolizumab has been heralded as a new era in the treatment of NSCLC and is now recommended as front-line therapy for advanced stage disease where the level of PD-L1 is 50% or greater. This treatment is active in both squamous and non-squamous histology. Pembrolizumab was approved by the FDA in October 2016 and by the European Commission in January 2017.
Conclusion
NSCLC survival is gradually improving, but the majority of patients will die from their cancers as they usually have advanced disease at diagnosis. In the last 20 years, considerable progress has been made in staging and selecting treatments based on tumor biology and patient characteristics and preferences.
Technical improvements in planning and delivery of radiotherapy with SABR, 3D conformal techniques, IMRT and respiratory gating mean that higher doses can be delivered to the tumors while minimizing significant damage to adjacent tissue. This in turn can improve the likelihood of long-term control and cure for early-stage tumors.
The major gains in lung cancer survival have come from improved treatment of early-stage or localized (in the chest) lung cancer. The big challenge currently is that of improving treatment of advanced stage cancers. Very few patients survive beyond two years. With the advent of biological therapy in addition to chemotherapy, there are now more options for both first-line and subsequent courses of treatment. The latest practice-changing development has been the introduction of immunotherapy as first-line therapy for advanced disease.
We are now in the era of being able to provide personalized cancer care for the full range of NSCLC, providing real hope for the future.