Neurotechnology covers a huge field of medicine, which is rapidly evolving through innovative and exciting scientific advances.
The technology, which is defined as “the application of engineering principles to the understanding, engagement, and repair of the human nervous system,” is already being used to restore hearing in deaf patients, help with movement disorder in Parkinson’s disease, and restore sight in patients with degenerative retinal disease.
This article will look at the latest innovations in neurotechnology that are likely to impact insurance underwriting and claims outcomes in neurological diseases and disorders in the future.
New technologies
- Neural interfaces are promising technologies that may have the potential to impact millions of people worldwide who suffer from particular neurological diseases. These interfaces are electronic devices that interact with the nervous system to record and/or initiate particular activities. They are already being used in retinal prosthetics to help restore visual acuity in people with age-related macular degeneration (AMD), to aid stroke recovery, to enhance sound through cochlear implants, and to boost memory and concentration through non-invasive brain stimulation techniques. Such devices could enable disability claimants to return to work or carry out activities of daily living that were previously difficult, if not impossible, to complete.[1,2]
- Brain-machine interfaces (BMIs) are neural interfaces that allow the brain to communicate directly with a machine. The neurotechnology company Neuralink, co-founded by Elon Musk, is currently developing a chip that can be implanted in a person’s brain to record electrical signals. Micron-size threads containing electrodes are inserted into areas of the brain and connected to an implant called the Link. The Link collects electrical signals and sends the information via Bluetooth to the Neuralink app on a mobile device. The technology is designed to enable the user to directly control devices such as a smartphone or computer by just thinking about it.[3]
- Human Machine Teaming (HUMAT) is a technology wherein neuroadaptive systems adapt to an operator’s mindset through the real-time analysis of brain activity. This could lead to teammates instantaneously adapting to each other’s thought processes and intentions. These technologies are still in development and not yet ready for human clinical trials.[2] Furthermore, technology that interacts with the brain raises ethical concerns regarding data privacy, and it is critical that any technology that gathers medical data be managed carefully.
Several clinical trials are testing the ability of brain implants to record brain signals and feed them wirelessly to a computer device, allowing people with conditions such as complete locked-in syndrome (CLIS) and amyotrophic lateral sclerosis (ALS) to communicate. Research has shown that patients with ALS who have retained eye movement capability are able to use BMI technology to communicate, but patients with CLIS are not due to full-body paralysis. Otherwise known as experimental electrocorticography (ECoG) sensing, implanted electrodes establish a direct link for transmitting information between the brain and the implanted BMI. The aim is to help people with severely debilitating neurological conditions communicate as well as improve their motor function where possible.[4,5] From the insurance perspective, the potential benefits for policyholders with these conditions who are receiving long-term care are clearly significant.
Neurotechnology is also being used to restore motor function in people with complete spinal cord injuries. Following such an injury, nerves in the spinal cord are frequently unable to transmit neural impulses to create movement, but patients may have some neural connections that still function. A recent study showed that inserting a paddle-shaped device embedded with electrodes into the remaining healthy spinal cord enabled several patients to walk. Wires from the electrodes were connected to a neurostimulator implanted under the skin in the abdomen. Three men aged 29 to 41 participated in the study. Independent stepping or walking was restored by day one, but this included having over 60% of their bodyweight supported during the process. While this new technology has demonstrated the ability to improve motor function, at least six centimeters of healthy spinal cord must be available to implant the electrodes, meaning that this technology is not suitable for all paralyzed patients.[6]
Additional medical technologies
- Peripheral nerve injury occurs in approximately 2-5% of all trauma cases and can have long-term consequences for patients. Bioresorbable electronic medicine consists of the development of biodegradable and bioresorbable wireless devices that can speed up nerve regeneration. These devices are implanted into patients to stimulate nerve repair, but then dissolve in 24 to 48 hours. The direct electrical stimulation of injured nerves available from these devices has been shown to accelerate recovery, complementing existing surgical techniques.[7,8]
- Microscopic implantable devices that change certain electrical signals in nerves are also being developed to treat rheumatoid arthritis (RA) and other inflammatory disorders. More than 400,000 people in the U.K. currently suffer from RA, and a third of newly diagnosed RA patients have to stop work within two years due to chronic symptoms. Known as bioelectronic medicine, this emerging field of neurotechnology uses electrical devices to read and understand the signals that pass through the peripheral nervous system. The device is placed on the vagus nerve in the neck and inhibits cytokine production to reduce inflammation. Insurance claimants could benefit significantly from this technology through reduced pain levels and improved quality of life.[2,9]
- Examples of other neurotechnological innovations include a deep brain stimulator, essentially a miniaturized brain pacemaker that could help treat diseases such as Parkinson’s disease, resistant hypertension, stroke, Alzheimer’s disease, and vascular dementia. One third the size of existing brain stimulation devices, it requires only one surgical procedure to implant the device, unlike existing devices which require more. This type of deep brain stimulation has been shown to almost completely stop tremors caused by Parkinson’s disease and to reduce the chance of epileptic seizures in patients.[2]
- Transcranial magnetic stimulation (TMS) is a non-invasive outpatient treatment option that uses magnets to stimulate the brain and alleviate symptoms of severe depression. Another concept, neurofeedback (NFB), uses computer technology to analyse and visualize brain activity on screen in real time to treat depression, epilepsy, chronic pain, attention deficit hyperactivity disorder and insomnia. This real-time visualization allows a person to modify their behavior towards an agreed “set point,” giving them greater stability in managing the disorder. One recent study showed that 75% of patients reported a reduction in pain using this technique.[2] Given the rising incidence of depression worldwide, this type of treatment could help thousands of people suffering from this and other mental health disorders.
Experimental surgery
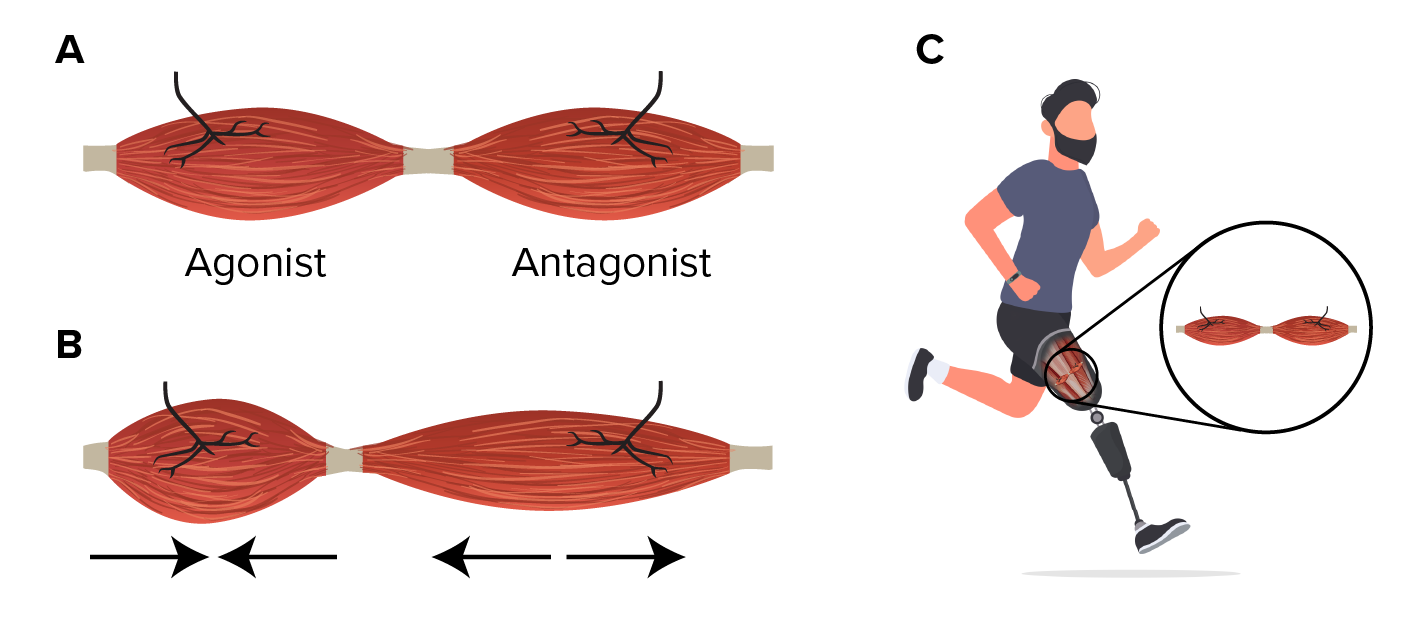
A new experimental procedure in amputation surgery is helping to restore proprioception, the brain’s ability to sense where its body parts are in space and how quickly they move. The new technique recreates the connections between muscles that are lost in the current method of amputation surgery. Proprioception works when muscles are paired up, with one contracting and the other stretching.
The procedure, known as agonist-antagonist myoneural interface (AMI) surgery, joins opposing muscles follow amputation, allowing for physical agonist-antagonist muscle relationships to enable greater motor control and preserving proprioceptive sensorimotor neurophysiology. A new 10-patient clinical trial in Maryland, U.S., seeks to help patients’ brains superimpose a phantom limb over a prosthetic one and allow them to perceive the prosthetic limb as their own.[10,11]
Figure 1:
Summary
Developments in neurotechnology have advanced significantly as scientists’ understanding of how the brain functions and how technology can interact with brain waves has progressed. This enhanced understanding is creating new approaches to treat severe or advanced neurological disorders, allowing patients to live longer and healthier lives. These technologies could change the course of medicine and allow people greater access to insurance cover. Claims for long-term disability or long-term care benefit could be reduced and, more importantly, more people could return to work and carry out activities of daily living.