Candida auris (C. auris) is a recently emerged multi-drug resistant fungus. Its four main strains have adapted swiftly since the first was detected and identified in Japan more than a decade ago.
At this point, it has spread around the world and is most problematic in high-dependency healthcare environments such as hospital intensive care units (ICUs) and nursing homes. Concerns about C. auris reflect rising worries about antimicrobial resistance (AMR), a worldwide health threat which researchers believe could potentially cause up to 10 million deaths per year by 2050.1, 3 With one in five deaths worldwide already caused by sepsis (stemming in some cases from candidiasis),10 this 10 million statistic cannot be ignored.
The past several decades have seen microbes become increasingly resistant to the modern arsenal of antimicrobial drugs, due in large part to misuse and overuse of antibiotics in medicine and animal agriculture. The early 20th century saw great advances in understanding and controlling infectious disease, but research for more than a half-century has focused largely on understanding and treating cancer, cardiovascular conditions, autoimmune diseases (e.g., multiple sclerosis and rheumatoid arthritis), and chronic diseases such as type 2 diabetes. The problem is compounded by the fact that few new antimicrobial drugs have been discovered or developed in the last 25 years.11
This article will discuss the emerging Candida strain of C. auris and its growing impact on healthcare and insurers.
What is Candida, and What is Candida auris?
Candida is a well-known fungus with multiple strains, the majority of which live harmlessly on the skin and inside the human body and are kept in check by co-existing bacteria in the microbiome. Candidiasis, the fungal infections it causes, is most commonly seen in the mouth and throat, the gastrointestinal tract, the vagina, and in skin folds. (Yes, diaper/nappy rash is a form of candidiasis.)
Invasive candidiasis happens when Candida enters the bloodstream or internal organs, which can occur during surgeries and invasive procedures such as catheterization, intubation, and tracheostomies.
Broad-spectrum antibiotics and overuse of antifungals, as well as the oral steroid medications that treat asthma and autoimmune conditions, can leave the body vulnerable to Candida infections. Corticosteroids weaken the immune system, allowing fungal infections to take hold. Diabetic individuals are also very susceptible to fungal infections due to altered functions of their immune systems, particularly in those with poor glycemic control, or as a direct effect of their elevated blood glucose levels, which encourages fungal colonization.
C. auris is a newer species of Candida. First identified and named in 2009, it causes bloodstream and intra-abdominal infection in surgical, intensive care, and other high- dependency healthcare situations, and is the first known species of Candida to be resistant to nearly every existing treatment. Indeed, some strains are already resistant to all currently available treatments, which in 2018 caused it to appear on the U.S. Centers for Disease Control and Prevention (CDC) list of urgent threats4 as well as its list of nationally notifiable diseases (meaning that occurrences must, by law, be reported to government authorities).
Where Is It From, and Where Is It Now?
The manner of C. auris’ evolution and how it behaves are most interesting. One of its unusual aspects is that its four known strains, or clades, appear to have emerged at approximately the same time in four widely-flung locations: east Asia, south Asia, southern Africa, and South America. Upon sequencing the genomes of C. auris taken from each location, it was found that the four clades have enough genetic similarities that they may have come from the same ancestor, but they are also genetically distinct from one another. This has led to a hypothesis that C. auris may have developed independently and simultaneously in multiple regions.6
Possible explanations offered as to how the separate clades emerged and evolved range from overuse of anti-fungal preparations in humans and livestock to climate change.8,13 Despite the common assumption that fungi like warm, moist areas, few fungi can live, let alone grow, at the human internal body temperature of 37 degrees Celsius (98.6 degrees Fahrenheit), but C. auris has been shown to be able to live in temperatures as high as 42 degrees Celsius (107.6 degrees Fahrenheit).8
As little is currently known about C. auris’ initial genesis it is hard to say if climate change may have played a part, but it is an interesting hypothesis. Indeed, if the climate change hypothesis is valid, C. auris may be the first human pathogen to have taken advantage of it, evolving from an environmental fungus into one infectious to humans.9,13 Experiments have shown that certain fungi can readily adapt to growth at higher temperatures, and that fungal species in cities may be more thermotolerant than their rural counterparts. As C. auris is found on cooler human body locations, such as the skin and the ear canal, but not in the warmer gut, a theory put forth in 2019 posits that this fungus may have first adapted to warmer temperatures due to climate change. Its evolution into a human pathogen may have occurred by first becoming infectious to an animal host before mutating into human infectivity. It is interesting to note that the first human C. auris infection was found in the human ear, an area cooler than core body temperature.13 As the gap between internal human body temperature and ambient environmental temperature continues to narrow, new invasive fungal pathogens may also emerge.13 Further research will be needed to understand C. auris’ evolution.
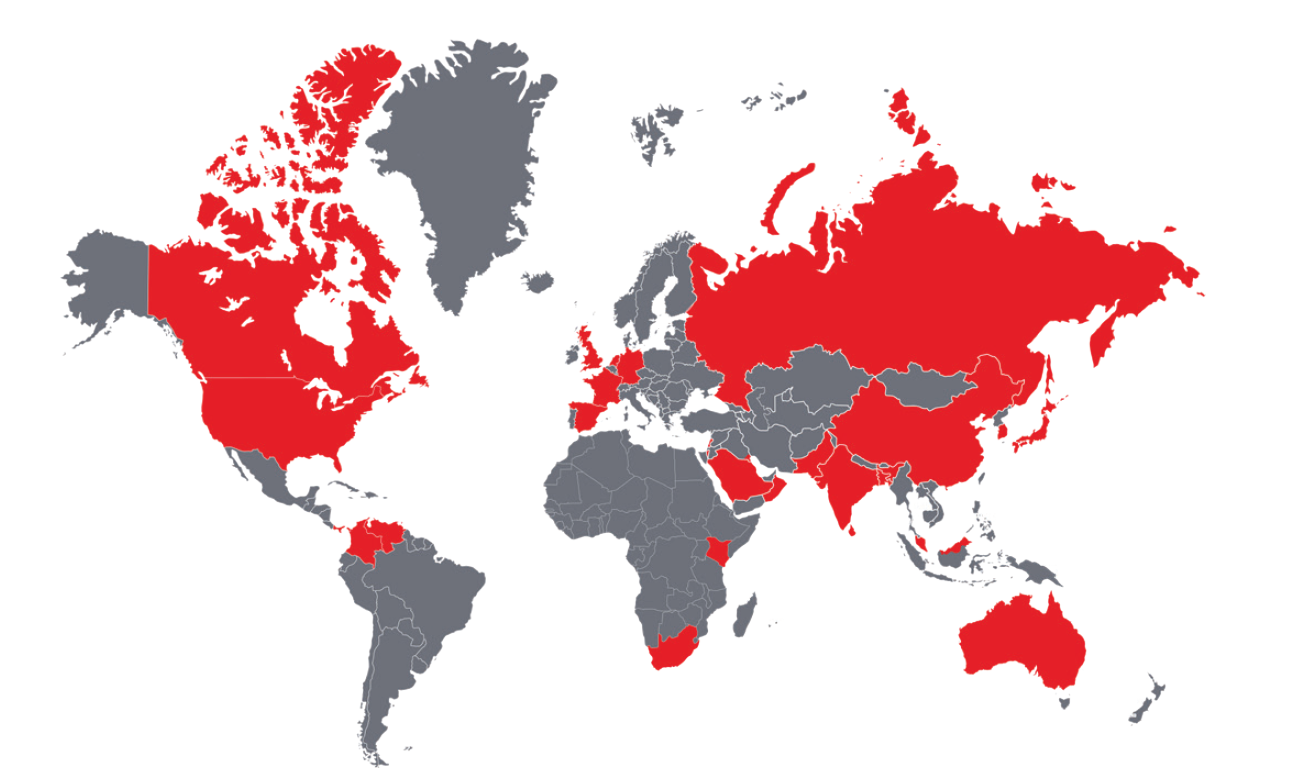
As for spread, single and multiple incidence of C. auris cases have been reported in more than 30 countries (see Figure 1). At the end of 2019, the U.S. alone had nearly 1,000 confirmed cases in 16 states, the majority of which were in New York, New Jersey, and Illinois. Some of these cases were found in individuals who had recently spent time in healthcare facilities in India, Kenya, Kuwait, Pakistan, South Africa, the United Arab Emirates, and Venezuela.12
Why the Interest Now?
Since 2009, C. auris has been spreading quickly around the world. The infections it causes are difficult to diagnose using routine fungal cultures and require more sophisticated molecular diagnostic methods. The infections can also be severe, affecting the bloodstream, surgical wounds, and not surprisingly, ears. It is known to have caused cases of endocarditis, osteomyelitis, and endophthalmitis.2
Symptoms of C. auris infection include fever and chills, sepsis, little to no patient improvement while on antifungal therapy, coma, and organ failure.
Total mortality from it is also high, with CDC estimates ranging from 30% to as high as 60%. However, many of those cases were among individuals who were already ill and in high-dependency healthcare facilities. It is not known yet if the mortality rate is any worse than other invasive Candida infections but what is different is that C. auris is causing outbreaks in healthcare settings to an extent not previously seen with fungi.
The biggest risk with C. auris is that it is resistant to treatment. In the U.S. alone, 90% of C. auris strains are already resistant to fluconazole, more than 40% are resistant to amphotericin B, approximately 2% are resistant to echinocandins,2 and a few C. auris strains are also known to be resistant to all three. Echinocandins are now the first line of defense against C. auris, but the few cases of its pan-resistance have occurred with people taking echinocandins, revealing a worrisome ability of C. auris to adapt quickly.
Transmissibility and Durability
Most invasive Candida infections are translocated from a patient’s own skin or abdominal tract. Unlike bacterial infections, most fungal infections, including most species of Candida, are rarely transmitted from patient to patient in healthcare settings, so it is not usually subject to routine infection control measures.2
C. auris, however, behaves differently. It is easily transmitted among patients in healthcare facilities and has the ability to both contaminate and persist in the environment and on surfaces. In one ICU outbreak in the U.K., reusable axillary temperature probes (skin surface thermometers) were found to be most likely responsible.2
C. auris is also more durable than other fungi: it is salt- resistant and able to survive on wet or dry surfaces and non-biotic surfaces for long periods of time.9 Specific anti- fungal agents must be used to decontaminate surfaces. From an infection control perspective, C. auris acts more like a multidrug-resistant, healthcare-associated bacteria than like a typical yeast.2 An outbreak of 50 cases in a London cardiothoracic center found persistent presence of C. auris around the bed-space areas. In the first group of identified U.S. cases, not only did C. auris colonies remain on the skin and other body sites weeks to months after their initial infection,7 the mattress, bedside table, bed rail, chair, and windowsill of the rooms where infected people had been placed were also found to still be contaminated one month after the infectious individuals had vacated.7
Who Is At Risk?
Individuals in good health are generally not susceptible to C. auris. More susceptible individuals tend to have: depleted immune systems; recently undergone surgery; open post-surgical wounds; lines/tubes or a tracheostomy; one or more chronic illnesses, such as diabetes; and/or taken broad-spectrum antibiotics or antifungal agents. At risk as well are individuals who have recently spent time in nursing homes, rehabilitation facilities, or other high-dependency care facilities where infected individuals were present.
Unfortunately, once a patient has been colonized with C. auris, the infection is very difficult to eradicate. This means infected people can spread it easily to other healthcare environments upon subsequent admissions. In addition, if they need more surgeries, they can develop serious complications.
Insurance Implications
It is unlikely a new life insurance applicant would have an active C. auris infection. However, insurers need to be alert to individuals who have had a recent surgery, had a recent extended stay in an overseas facility in a country where cases are endemic, or have a chronic health condition such as diabetes. Applicants may not have active infections but may still be colonized: some C. auris cases, according to the CDC, have gone undetected up to two years after hospitalization.
Current health status and any risk factors for persisting or re-emerging infective symptoms would need evaluating.
Caution should be applied in terms of disability cover, long-term healthcare and hospitalization products. Although those most at risk are already significantly unwell, some markets have a higher tolerance for sub-standard lives and there are some limited underwriting products with pre-existing conditions clauses. Since the risk factors can be vast, these clauses might not be entirely protective. In cases with recurrent or chronic Candida infections, further information and details may be required to fully assess the case.
On the claims side, given the high mortality rate of this microbe, especially from sepsis, death claims as well as health claims would need to be considered. There are implications for hospitalization and long-term healthcare covers that were purchased before chronic illness set in. A patient admitted for a scheduled standard surgical procedure may end up with an extended admission to the ICU that would cost much more than the original procedure.
Conclusion
C. auris is a new breed of pathogenic fungus. It is difficult to diagnose, treat, and eradicate. Its presence in healthcare facilities worldwide, especially nursing care, high-dependency care, and ICUs, is growing. More research is going to be needed to understand how this particular microbe is evolving and how best to deal with the global problem of antimicrobial-resistant pathogens, and this pathogen in particular.