Chronic pain is a major societal burden that not only impacts individuals but also carries a significant socioeconomic toll.
The prevalence of this condition across jurisdictions is approximately 20%. Chronic pain affects quality of life in many ways: through symptoms, functional limitations, social isolation, absenteeism, and possible disability.
The biopsychosocial model of illness – one that incorporates a condition’s associated physical, mental, and environmental aspects – is today the predominant and heuristic approach to managing chronic pain. However, this model is often overlooked by pain medicine practitioners, who frequently treat pain only with biological means: medications, injections, invasive procedures, and surgeries that target the mechanisms suspected of causing the specific pain on neurological, physiological, or anatomical levels. The subtleties of how pain is expressed can frequently be lost to the expected outcome of applying precision-targeted medical treatments when what is truly sought by patients is relief of suffering, of which pain is but one component.
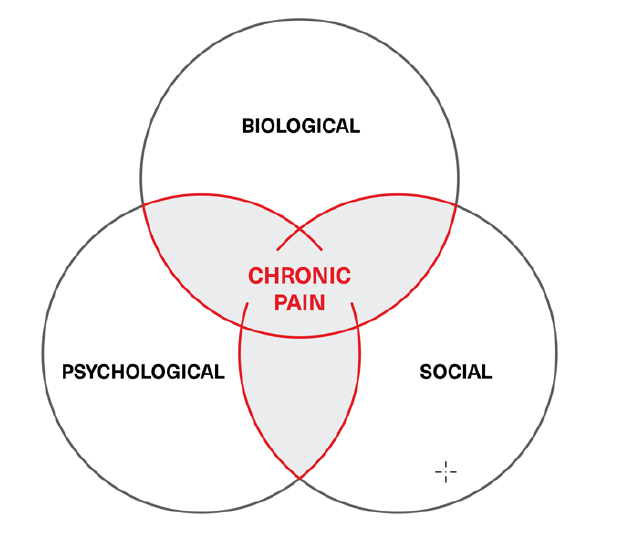
The biopsychosocial model of chronic pain is usually depicted as the Venn diagram in Figure 1.
Figure 1:
Chronic pain biopsychosocial model
Medicine generally renders the treatment of human ailments as scientific endeavors, and for the most part this approach has worked. Experimentation, research, and clinical trials have usually been able to tease out many of the intricacies of human pathophysiology, enabling the development of effective treatments for many diseases. Relevant to the focus of this article, research has also shown the role glial cells play in basic aspects of chronic pain. Where the scientific method has faltered, however, has been in addressing non-biological expressions of pain and illness.
What should be clear from the biopsychosocial model (Figure 1) is that mental health plays a crucial role in the evolution and course of chronic pain states. Its impacts are demonstrated by the frequent concurrence of depression and anxiety with chronic pain. Depending on the data source, 70% to 85% of chronic pain sufferers may also have significant mental conditions; most often, depression. In addition, between 15% and 100% (mean 65%)19 of those diagnosed with depression may also exhibit significant pain symptoms. These numbers demonstrate a compelling etiological commonality, and glial cells may play a role.
Glial Cells and Mental Health
There is no arguing that exposure to trauma, whether physical or mental, can impact an individual. Physical trauma can engender aberrant physiological processes that may lead to chronic pain, and exposure to psychological stressors can also trigger responses that may lead to pathological mental states such as depression and post-traumatic stress disorder (PTSD). The similarities between these two constructs are reflected in individual outcomes: not everyone who experiences trauma or stressors develops chronic pain and/or psychiatric morbidity. The underlying mechanism for triggering chronicity in both pain symptomatology and pathological mental states may lie with the neuroinflammatory role of glial cells.
Research has determined that oligodendrocytes, the cells that make the protective myelin sheaths that surround neurons and preserve nerve conductivity, are demonstrably fewer in number and generate irregular myelin coverage in brain tissues of mice that displayed social withdrawal after exposure to aggressive stressors. Mice that did not develop social withdrawal behavior and continued to interact normally showed healthy myelin and relatively abundant oligodendrocytes.1, 2, 3, 4
Research has also demonstrated that symptoms of depression can enhance inflammatory responses. This effect can specifically be seen in the production of interleukin-6 (IL-6), a proinflammatory cytokine.5 As acute stress can result in measurable transient increases in systemic inflammation, a chronic depressive state can also sensitize an inflammatory stress response. In chronic pain, the process of central and peripheral sensitization propagated by neuroinflammatory glial cell activity also implicates IL-6 as a key regulatory mechanism. Thus, both chronic pain and depression (and most likely other impaired mental health states) are mirrored and accentuated through symptom magnification and physiological augmentation by a common inflammatory pathway.
Case Study: Fibromyalgia
Fibromyalgia is one of the most difficult and frustrating chronic pain conditions to assess and treat. It is considered a “functional pain syndrome,” in that it presents as pain with no obvious organic origin.20 Because of this, fibromyalgia can be an effective model for demonstrating the biopsychosocial paradigm set forth in Figure 1.
A fibromyalgia diagnosis is one of exclusion, meaning it is arrived at in the absence of specific objective findings. Fibromyalgia is a condition which manifests with a variety of nonspecific symptoms, ranging from widespread musculoskeletal pain and fatigue to cognitive impairment, non-restorative sleep, and mood dysfunction. Given this broad constellation of potential symptoms, fibromyalgia can result in significant impairment for affected individuals. It is also a condition that arguably manifests in both a physical and mental context. It should additionally be noted that a diagnosis of fibromyalgia does not rule out the concurrent presence of other illnesses.
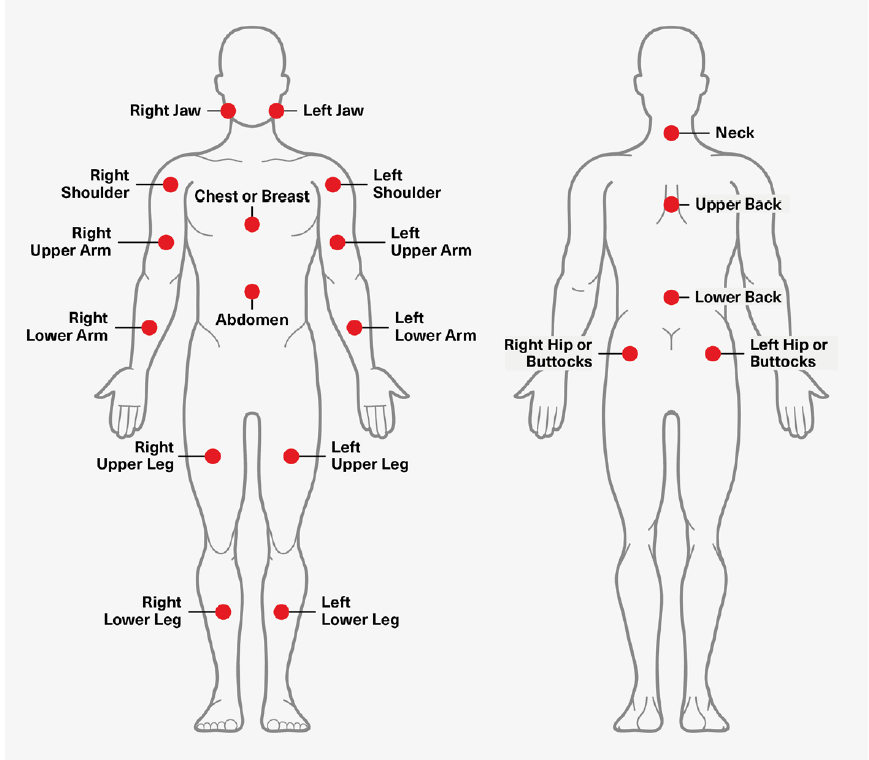
The currently used diagnostic framework for fibromyalgia, first developed in 2010 and revised in 2016, consists of the sum of two scores: the Widespread Pain Index (WPI) and the Symptom Severity Score (SSS). Developed by the American College of Rheumatology, the WPI scores fibromyalgia-related pain by assessing pain during the prior week in 19 specific places on the body. That assessment is to be done while taking current medicines and treatments and exempting symptoms from known diseases such as arthritis, lupus, and Sjogren’s syndrome. The numerical score, which consists of the total number of places where pain was experienced, is the WPI.21
Figure 2:
Adapted from: http://www.jlgh.org/Past-Issues/Volume-14-Issue-4/Ton-That_Fibromyalgia.aspx
The SSS is a two-part calculation. In the first part, the patient assesses the severity of three categories of fibromyalgia symptoms: fatigue, waking unrefreshed, and cognitive symptoms (e.g., forgetfulness, concentration difficulties, mental slowness, language-related problems, reduced planning/organizational abilities). In the second, patients indicate which of a checklist of 41 non-fibromyalgia-related conditions and symptoms they are currently experiencing. The number of “yes” answers are then matched to a scoring range from 0 to 3.21
The totals of the WPI and SSS, once added together, yield an overall score that is today the accepted clinical tool for diagnosing fibromyalgia. However, each score’s weight ultimately affects the final numerical score. A patient meets the diagnostic criteria for fibromyalgia if the following conditions are met:
- The WPI score (Part 1) is ≥7 AND the SSS (2a + 2b) is ≥5; OR the WPI score is from 4 to 6 AND the SSS is ≥9. 22, 23
- Generalized pain is present in four of the five regions depicted in Figure 3.
- Symptoms and conditions have been present at a similar level for at least three months.
- There is no other disorder present that would otherwise explain the pain.
For example, if a patient’s WPI score was 9 and SSS was 6, he or she would meet the diagnostic criteria. However: If the WPI was 5 and the SSS was 7, the patient would not.
Figure 3:
Assessing Generalized Pain Regions
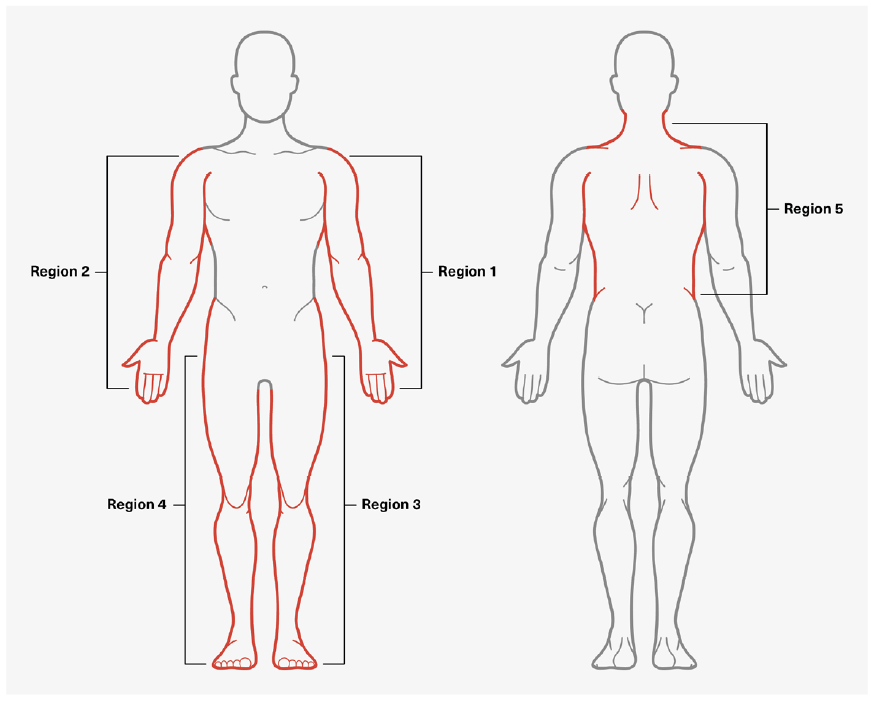
Adapted from: http://www.jlgh.org/Past-Issues/Volume-14-Issue-4/Ton-That_Fibromyalgia.aspx
Etiological Mechanisms
Although the symptomatology of fibromyalgia has become much clearer over the years, its underlying pathophysiology is still not well understood. Currently, central sensitization of the nervous system is a widely accepted foundation for fibromyalgia’s clinical presentation. Other identifying mechanisms include aberrant activity of the Diffuse Noxious Inhibitory Controls (DNIC), which is the ascending spinal pathway for pain sensation. Common to both central sensitization and DNIC malfunction is the link of neuronal activity, which would necessarily implicate glial cells and their activation as a component of the induction and perpetuation of chronic pain.
Recent research using positron emission tomography (PET) scan imaging studies has demonstrated that glial activation is present in fibromyalgia patients.6 This finding is consistent with the association of fibromyalgia syndrome as a neuroinflammatory event. Translational research is also implicating tumor necrosis factor alpha (TNF-α), which is generated by the microglia, as a key pathological factor in fibromyalgia.7 The research further underlines the concurrence of mental health manifestations arising from the same neuroinflammatory process. Herein lies the inextricable connection within fibromyalgia of the duality of medical states affecting the individual. It is not unreasonable to extrapolate this occurrence to other chronic pain conditions.
Treatments
Treatment of fibromyalgia in an ideal setting is holistic, encompassing multiple medical therapy modalities and psychological interventions. Several classes of medications are currently used which now, in the context of research findings, can be rationalized by their mechanisms of actions on glial cell function.
Antidepressant medications such as the serotonin-norepinephrine reuptake inhibitors (SNRIs) duloxetine and milnacipran, while acting primarily on neurotransmitter function, may also modulate glial function.6 Older tricyclic antidepressants such as nortriptyline and amitriptyline may also have a modulating effect. Using antidepressants to simultaneously treat chronic pain and depression targets the etiological mechanism common to both.
Anticonvulsants such as pregabalin and gabapentin dial back neuronal excitability, thereby modulating central sensitization and inhibitory controls (DNIC). Pregabalin has been shown in animal studies to demonstrate neuroprotective action and decrease proliferation of astrocytes in spinal cord damage.8 Animal studies on multiple sclerosis also show that pregabalin enhances myelin repair and dampens glial cell activation.9 It may thus be no coincidence that pregabalin is often used to augment antidepressant therapy.
The use of an opioid antagonist in fibromyalgia is based on the assumption that its actions inhibit glial activation, thereby mitigating the neuroinflammatory process. Low-dose naltrexone can be custom- compounded into capsules to provide a dose far lower than the usual therapeutic dose. Typically, the action of naltrexone is to block opioid effects by competitive binding to the receptor and impacts the endogenous opioid effect, a mechanism postulated to be behind its efficacy in the treatment of alcohol use disorder.
In addition to medical therapy, psychological therapy modalities have also been validated for fibromyalgia relief in numerous therapeutic trials. Cognitive behavioral therapy (CBT), the most often used approach, has been found to be at least as effective as medical therapy. The foundation of CBT is that thoughts, feelings, emotions, and physical sensations are interconnected, and the persistence of negative feelings and thoughts may cause an individual to manifest aberrant behavior as well as engender physical symptoms such as pain. CBT is a short-term, problem-focused approach to dealing with an individual’s internal cognitive-emotive processes and external stimuli. Along the same lines, mindfulness- based meditation and acceptance-commitment therapy (ACT) can also engage an individual’s cognitive landscape as a means to ease physical sensations of pain.
Ultimately, what is clear is that strictly medical approaches cannot hope to deal with the effects of external factors that push and pull an individual through reflex actions, patterned behavior, and maladaptive responses, ultimately affecting clinical outcomes.
Conclusion
Will targeting glial cells successfully treat chronic pain and mental health conditions? In this review, two aspects of the biopsychosocial model and the common link of glial cell activity were discussed in an effort to address this question. Fibromyalgia is an example of a condition which demonstrates that despite finding specific pathophysiological mechanisms for treatment, the whole-person approach, addressing both medical and psychosocial factors, may ultimately enable a patient to overcome many of the physical manifestations of this illness.
Targeting glial cell function in chronic pain states may well refine a more encompassing treatment modality, but the multiple impacts of sociocultural influences on the other two domains of the biopsychosocial model cannot be easily measured in objective terms, nor can they be readily treated with medical therapy.
As for the assertion that chronic pain should be considered a disease in its own right, arguments will likely be raised regarding whether to classify pain as a primary or secondary manifestation of an illness within the context of pathophysiological mechanisms. Arguments might also be made that pain cannot beget pain, and thus the criterion for classifying pain as a disease may be lost to circular reasoning.10 Clinicians will argue otherwise, but this debate is for another time and place.
When the mechanisms and complex etiologies of chronic and persistent pain states become more fully understood, underwriters, claims assessors, and insurance medical directors will be better positioned to assess risk, manage claims, and even potentially provide products or interventions to contribute to the restoration of health for those who experience chronic pain.