Key takeaways
- Artificial intelligence (AI) is revolutionizing lung cancer screening by improving detection rates and risk prediction.
- Virtual biopsies are emerging as non-invasive methods to assess tumor characteristics and clinical outcomes, potentially replacing the need for surgical biopsies and enabling more personalized care.
- While AI and advanced imaging techniques offer improved sensitivity in lung cancer detection, they also present challenges, necessitating careful implementation and ongoing evaluation to balance their benefits and risks in clinical practice.
Despite advances in lung cancer treatment, especially targeted therapies for genetic mutations, the disease remains one of deadliest in the world. Lung cancer is often diagnosed at an advanced stage, as most patients do not develop symptoms until late in the disease. In 2022, lung cancer caused 1.23 million deaths globally, accounting for 18.7% of all cancer fatalities.1
As with most cancers, earlier diagnosis leads to better outcomes. To drive earlier detection, low-dose computed tomography (LDCT) scanning has become the most widely used lung cancer screening tool in use today.
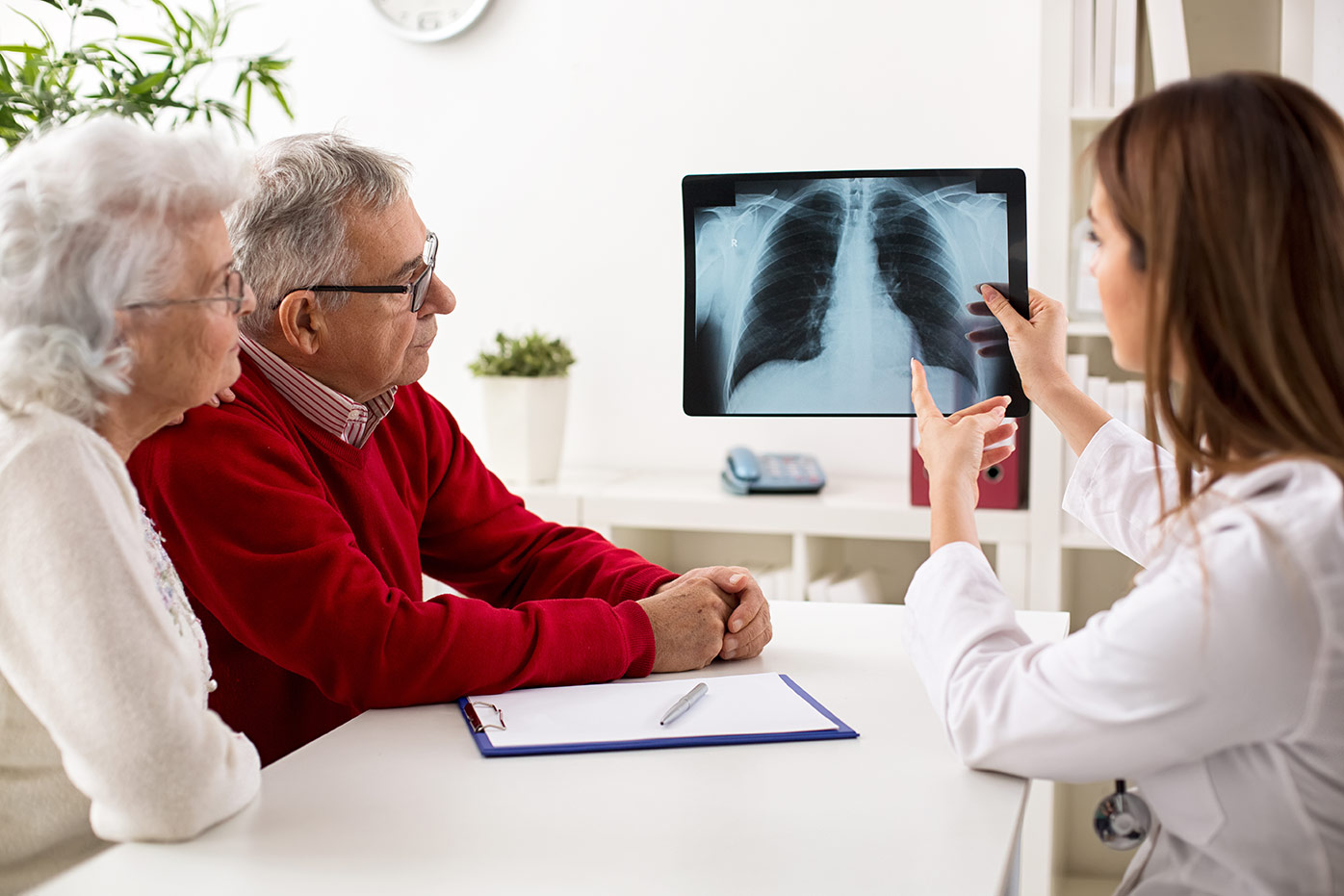
Since LDCT was introduced in the US for high-risk individuals in 2011, the proportion of cases diagnosed at a localized stage has steadily increased. By 2016, for the first time, localized diagnoses surpassed those diagnosed at the regional stage.2 Expanded screening could increase early-stage lung cancer diagnoses by up to 70%, as well as lead to the detection of more cases of carcinoma in situ and other precursor lesions.3,5 Notably, chest radiography (x-rays), with or without sputum cytologic examination, showed no improvement in patient outcomes.6
Trials showed that LDCT screening reduces lung cancer mortality in smokers by 20% to 24%. However, it comes with challenges, both clinically and in the context of risk assessment.
From a clinical perspective, implementation of LDCT lung cancer screening requires resources, systems, and processes to be put in place to manage the findings – particularly uncertain results – effectively.3 Additionally, there are personal and economic implications associated with rolling out screening programs.
Another key consideration is the risk of cumulative radiation exposure, particularly for patients requiring ongoing surveillance of indeterminate nodules. Ultra-low-dose computed tomography (ULDCT) reduces radiation exposure of 0.22mSv vs. 1.5mSv with LDCT. Combined with image reconstruction algorithms, ULDCT significantly mitigates radiation exposure risk while maintaining high-quality imaging for solid nodules larger than 5mm. However, for smaller and non-solid nodules, further optimization may be required, limiting both the clinical and risk assessment benefits of this imaging advancement at the current time. Another advancement, photon counting detector CT (PCD-CT), offers the potential to further reduce radiation exposure with even more enhanced image resolution.7
Through an insurance lens
From an insurance perspective, three key considerations stand out in LDCT lung cancer screening. The first is assessing the likelihood of current or future malignancy in a detected lung nodule. Most underwriting guidelines take a risk-based approach, evaluating both nodule characteristics and an applicant’s individual risk factors. In high-risk groups, LDCT frequently detects lung nodules, yet only a small percentage eventually prove to be malignant.3,4 False-positive rates can reach up to 13%. To address this, standardized algorithms in radiology reporting have been developed to reduce discrepancies. Prudent risk assessment must balance clinical protocols, disease prevalence, likelihood of malignancy, and risk appetite in the event of positive findings.
Beyond identifying high-risk nodules, LDCT screening frequently detects incidental findings that may require further clinical evaluation and risk assessment. The rate at which these findings are reported varies significantly, ranging from 23% in community centers to 67% in university settings. Despite these differences, initial lung cancer screening trials found that fewer than 10% of incidental findings were clinically significant, with coronary artery calcification and emphysema being the most prevalent and relevant.3 Both conditions are independent risk factors for overall mortality and lung cancer, necessitating follow-up and further assessment.
Interstitial lung disease (ILD) is another significant incidental finding due to its potential impact on mortality and morbidity, also requiring further follow-up. Other lung-related incidental findings include bronchiectasis, tuberculosis, and pleural plaques/thickening and effusions. Osteopenia and osteoporosis may also be detected, although bone density is not always reported. If identified – particularly with associated vertebral fracture – they become noteworthy from a risk assessment perspective. As reporting standards for incidental findings evolve, they will help clarify the clinical and risk assessment significance of these findings.
Finally, overdiagnosis remains a concern from both an incidence rate trend and critical illness perspective, owing to increased claims and possible need for definition review.3 Quantifying overdiagnosis is challenging and may be underestimated in randomized population-level trials. Still, data from lung cancer screening trials provide some insight. In the National Lung Screening Trial (NLST), the probability that lung cancer detected by LDCT was an overdiagnosis was estimated at 18.5% initially after a mean follow-up of 4.5 years, decreasing to 3% after 9 years. The NELSON trial reported similar findings, with estimates of 19.7% and 8.9% at 4.5 and 5.5 years after final screening.3
Minimizing overdiagnosis – and the resulting overutilization and overtreatment – is essential. However, when screening occurs outside of structured population screening protocols, the impact becomes more difficult to quantify.