As of 2020, colorectal cancer (CRC) was the second most common type of cancer diagnosed and the third leading cause of cancer-related mortality.
Like most other solid tumors, CRC is largely a consequence of progressive accumulation of abnormalities in tumor suppressor genes and oncogenes, which initiate and sequentially promote carcinogenesis.
More than 90% of CRCs are adenocarcinomas. Like many other solid tumors, CRC is a heterogenous disease with different subtypes that are distinguished by distinct clinical and molecular features. CRC originates in the glandular epithelium (colonocytes) of the large bowel. The process begins with an index genetic aberration in healthy epithelium, which gives the affected colonocyte a selective growth advantage and the opportunity for clonal expansion of cells at that site. These cells then proliferate in the colon mucosa and ultimately present as a polyp.
The initiating mutation of CRC usually occurs in the APC tumor suppressor gene, which is on chromosome 5. This gene participates in a number of functions, including suppression of abnormal cell proliferation. The silencing or inactivation of this gene results in aberrant functioning of the Wnt signaling pathway, a critical and highly conserved evolutionary pathway in animals that regulates cell communication, division, differentiation, and gene transcription.
An APC mutation is usually a spontaneous event at a particular site in the colon, but the causative chromosomal abnormality may be an inherited germline mutation. Germline mutations create a field defect in all colonocytes which exposes the entire colon to an increased risk of cancer. The APC germline mutation is responsible for familial adenomatous polyposis (FAP) syndrome, a rare type of inherited cancer predisposition syndrome characterized by hundreds to thousands of precancerous colorectal polyps (adenomatous polyps) which, if not removed, usually result in CRC at a young age.
The transformation from an initially benign expansion of colonocytes into entities with increasing growth perturbation requires the sequential acquisition of additional genetic abnormalities. This accumulation of serial molecular alterations establishes a momentum of genetic instability, which results in affected cells developing increasingly abnormal characteristics leading to a final transformation into invasive cancer.
Most successful cancers contain many genetic abnormalities. It is estimated that an accumulation of 10 or 15 “hits” (i.e., abnormalities) in key signaling pathways is necessary for a cancer to establish. This process must begin during the short lifespan of a normal colonocyte. Each sequentially acquired abnormality provides affected cells with an incremental growth advantage which further enhances chromosomal instability and facilitates the neoplastic process.
Environmental factors are also established promoters of genetic instability and are known to affect gene structure and function. These factors may act as direct mutagens or exert adverse influences on gene expression. In addition, visceral obesity, smoking, lack of physical activity, alcohol consumption, western dietary patterns, and alterations in the colon microbiome are established promoters of carcinogenesis. All of these epigenetic factors influence the prevalence of sporadic cancers and may be responsible for familial clustering that can be seen in the absence of transmissible germline mutations. Most cancers occur in the absence of any family history while others have familial clustering as noted above.
Familial CRC clusters may result from a heritable germline mutation, such as FAP and Lynch syndrome (hereditary non-polyposis colorectal cancer [HNPCC]), although a substantial proportion are still the result of acquired mutations, possibly driven by common environmental exposures or other epigenetic influences. Epigenetic factors also play a role in the expression and penetrance of cancer in those with an identifiable genetic mutation. (Figure 1)
Figure 1:
Proportions of Colorectal Cancers Associated with Sporadic and Hereditary Factors
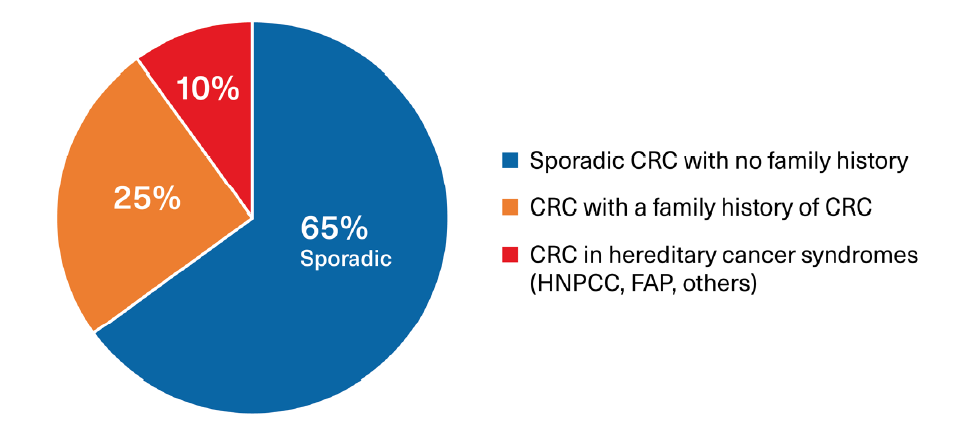
Source: RGA
A family history of CRC increases individual cancer risk considerably. Indeed, the risk of cancer is doubled in those with one affected first-degree relative and quadrupled in those with at least two affected first-degree relatives.
Normal colon epithelium is renewed every few days, as older cells undergo apoptosis (programmed cell death) and are shed. The colon cell proliferation rate is of the order of 3-10 billion cells each day and this high turnover provides an enormous potential for the accumulation of genetic and epigenetic changes. Normal cell growth and division for tissue repair and organ maintenance is governed by genetically determined growth signals. Proto- oncogenes promote tissue growth while tumor suppressor genes inhibit cell proliferation and growth expansion. Mutations in proto-oncogenes, which cause them to be overexpressed, and mutations in tumor suppression genes which cause them to be silenced, are common promoters of colon tumorigenesis.
The DNA replication process occurring at cell division is surveilled by a DNA mismatch repair (MMR) system. This system identifies and repairs translation errors in newly synthesized DNA and limits material mutations. Defects in MMR promote the survival of spontaneous mutations, and also have a role in tumorigenesis which complements that of growth promoters. MMR genes are very prone to epigenetic influences.
It may take years for generations of cells to escape the body’s multiple regulatory layers that are designed to suppress carcinogenesis, and for normal epithelium to transform into a cancer and acquire potential for tissue invasion and metastasis.
The Molecular Basis of Colorectal Cancer
At least three major molecular pathways (mutator phenotypes) form the basis for CRC initiation and development. Each is activated or influenced by chromosomal aberrations. These three pathways still require the accumulation of sequential defects, and the pathways are not mutually exclusive. (Figure 2)
- Chromosomal Instability (CIN) pathway: CIN is identifiable in approximately 85% of sporadic colon cancers and refers to high rates of gains or losses of portions of chromosomes which affect gene expression. CIN tumors are recognized by mutations in tumor suppressor genes (such as APC), oncogenic growth promoter mutations (such as KRAS and BRAF), and mutations in genes such as TP53, which both inhibits tumor suppression and negatively influences apoptosis. These mutations all represent gains in function for the growth of cancer cells.
In CIN, the cancer pathway is commonly initiated by inactivation of the APC tumor suppressor gene, promoted by early appearance of a KRAF or BRAF promoter mutation, and then enhanced by the later acquisition of a TP53 mutation.
APC mutations have been identified in as many as 90% of sporadic CRC cases. In familial clusters of FAP syndrome, for example, all inherit a mutated copy of the APC tumor suppressor gene. - Microsatellite Instability (MSI) pathway: MSI is another type of chromosomal instability. It refers to the presence of high numbers and frequencies of very small replication errors in DNA scattered throughout the genome. The progressive and repetitive accumulation of these errors in a DNA sequence leads to a deficiency in the MMR system, which promotes a dramatic increase in mutation errors. MSI is identifiable in 15%-20% of sporadic CRC cases and is responsible for Lynch syndrome or HNPCC.
- Aberrant DNA Methylation pathway: Not all genes are expressed at all times. DNA methylation plays a crucial role in gene expression and is a signaling tool which may be used to lock genes in the on or off position. Methylation sites are distributed throughout the genome in what are called CpG islands. Methylation at these sites can change the gene’s expression and lead to gene silencing. Inappropriate silencing, particularly of tumor suppressor genes, may promote carcinogenesis. An abnormal CpG Island Methylator Phenotype (CIMP) is demonstrable in up to 30%-35% of sporadic CRC cases and is a characteristic of tumors which develop in polyps with a serrated morphology (discussed below). CIMP positive tumors are commonly associated with KRAS and BRAF mutations and often exhibit microsatellite instability.
Figure 2:
Molecular Pathways to Cancer
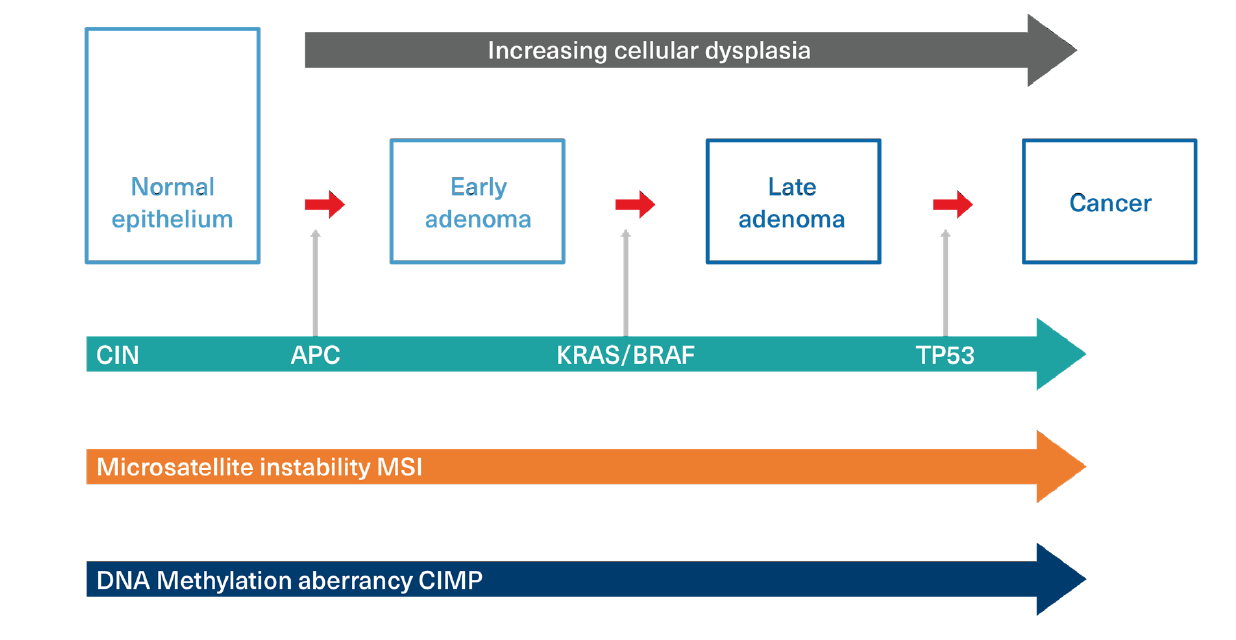
Adapted from: Malki A, et al. Molecular Mechanism of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2021; 22(1): 130.18
Nomenclature and Classification of Polyps and the Risk of Malignant Transformation
The acquisition of growth promotion characteristics in colonocytes results in the development of benign growths which expand and protrude into the bowel lumen in the form of polyps. While more than 90% of adenocarcinomas develop in benign polyps, most polyps do not harbor or acquire the requisite sequential carcinogenic mutations that promote transformation into overt malignancy.
The classification, terminology, and diagnostic criteria applied to polyps continue to evolve, particularly with respect to their molecular biology and potential for malignant change. An understanding of the various genetic pathways which induce and promote cancer transformation is central to polyp management and has led to the development of interventional strategies which have reduced CRC incidence and mortality.
The most recent classification of colorectal polyps appears in the World Health Organization (WHO) Classification of Tumors of the Digestive System, 5th Edition, published in 2019.
The prevalence of benign polyps increases with age. A significant increase generally begins between ages 40 and 50, predating the age-related incidence of invasive colorectal cancer (Figure 3).1 In polyps destined for malignant transformation, the sequence from a benign precursor to a cancer usually occurs over several years, with this lag period providing an ideal opportunity for early screening detection.
Figure 3:
Colon Cancer Incidence by Age – World
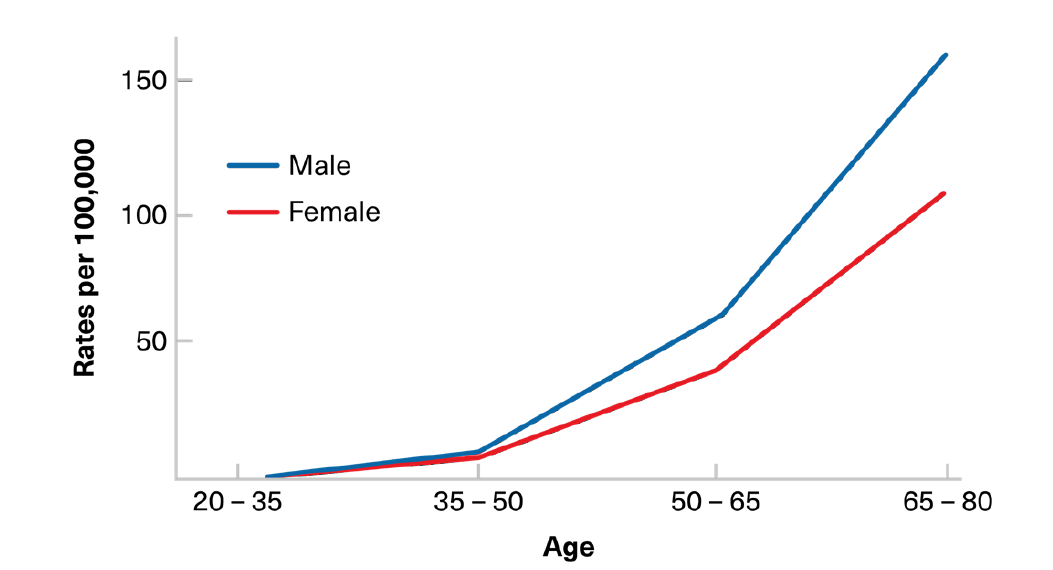
Adapted from: Ballinger AB, Anggiansah C. Colorectal Cancer. BMJ. 2007; 335: 715-8.
20Following detection and removal of an index polyp, subsequent surveillance intervals are governed by:
- Size (advanced size threshold defined as 10 mm)
- Number found (defined as >3)
- Classification (determined by histology)
- Presence or absence of any dysplastic features (determined by histology)
Morphological Descriptors
Two terms are used to describe the gross visual appearance of polyps found during a colonoscopy: Pedunculated (polyps with a stalk), and sessile (polyps that either have a broad base or are flat, with no stalk).
These gross features do not allow malignancy risk to be determined. Endoscopic removal is often easier for pedunculated polyps than for large sessile lesions.
That caveat is recognized in some follow-up surveillance guidelines discussed below.
Figure 4:
Pedunculated and Sessile Polyps
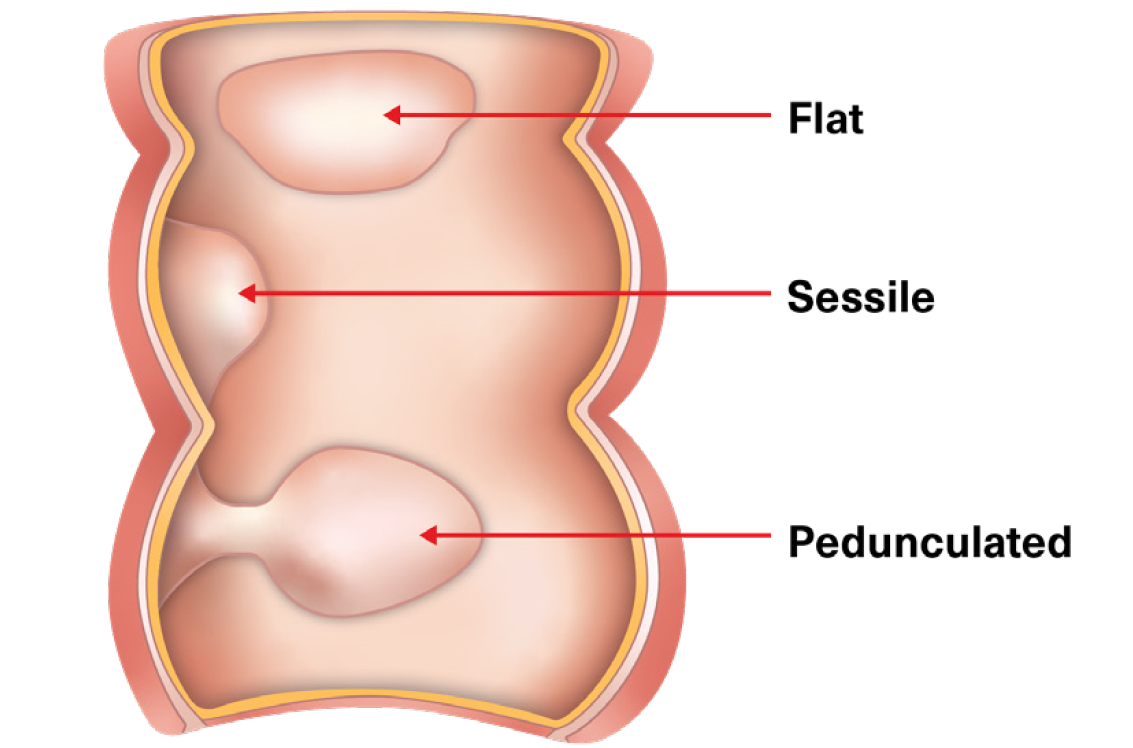
Adapted from: Walsh CL. Gastrointestinal Nursing. 2017 June; 15(5): 27.
Histological Classifications
Two polyp types, conventional adenomas and serrated polyps, are defined using two distinct histological features. These features are now known to align with different pathways to carcinogenesis.
Conventional adenomas (previously known as adenomas or adenomatous polyps), are the most common type. They represent the classic cancer precursor lesion and account for most CRCs.
Serrated polyps (previously known as hyperplastic polyps or metaplastic polyps) are less common and traditionally were not considered precancerous. They are now classifiable into histological subsets on the basis of morphological and molecular profiles which label some as having malignant potential. The term “serrated polyp” now encompasses a number of entities, often with subtle differences in architecture which may be difficult to distinguish. Hyperplastic polyps (HP), currently considered a subset of serrated polyps, are the most common serrated polyp type (75%) and have the lowest malignancy potential.
Table 1:
Polyp Types - Descriptors
Conventional adenomas | Serrated polyps |
Represent approximately 70% of all polyps | Represent approximately 30% of all polyps |
Defined by a “layered” appearance of epithelial cell growth | Defined by a “serrated” or “sawtooth” appearance of epithelial cell growth |
APC gene mutation is common (>90% of all adenomas) | Most remain classifiable as HPs with little or no malignant potential |
All have malignancy potential | Premalignant subsets of serrated polyps are now recognized |
Malignancy develops via a conventional pathway | BRAF (or KRAS) gene mutation is common in premalignant subsets (75%-90%) |
Malignancy risk is 10% | Malignancy develops via a “serrated pathway” |
Account for 85% - 90% of all sporadic CRC cases1 | Account for 10% - 15% of all sporadic CRC cases1 |
Figure 5:
Histology – Adenomatous and Serrated Polyps
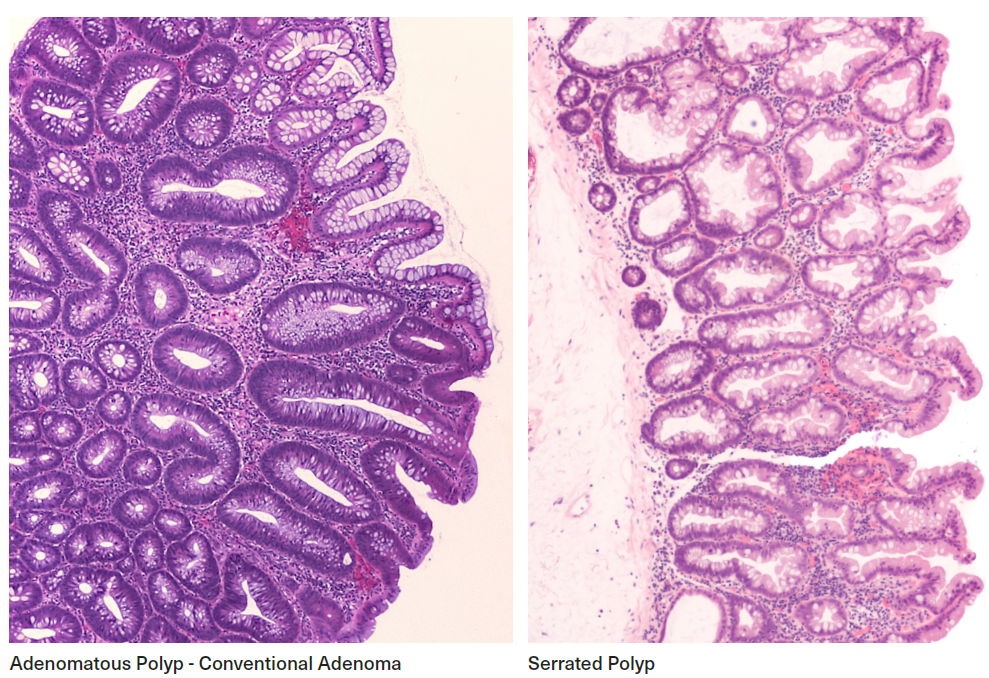
Table 2: Malignant Pathway Descriptors for Conventional Adenomas and Serrated Polyps |
Polyp pathway and type
| Pathway Descriptor* |
Conventional Pathway | Major carcinogenic chromosomal mutations |
Conventional Adenomas (75% of all CRCs) | |
Serrated pathway | Major carcinogenic chromosomal mutations |
Serrated Polyps (25% of all CRCs) | BRAF mutation common (80%) KRAS mutation less common (30% of traditional serrated adenomas) APC mutations less common (20% of traditional serrated adenomas)
|
*Mutational characteristics exposing polyps to cancerous change |
|
Description and Nomenclature
Conventional Adenomas
Conventional adenomas are the most common type of colon polyp. The term “conventional adenoma” is current nomenclature in surveillance guidelines and has replaced the older terms “adenomatous polyp” and “adenoma” in order to distinguish it from the adenomas now described in serrated lesion classifications. (The older nomenclature can still be found in some pathology reports.)
Most cancers arise in conventional adenomas and progress via a well-described “adenoma-carcinoma” sequence. Although only 10% of conventional adenomas will transform into malignancy, the majority harbor mutations in the APC cancer suppressor gene, a major gatekeeper against CRC. APC mutations are highly associated with both conventional adenoma development and malignant transformation. This transformation may be reflected in varying degrees of dysplasia on microscopy.
Conventional adenomas are the precursor polyps in most sporadic and familial CRCs and in those CRCs associated with heritable FAP and HNPCC syndromes. In sporadic CRC, APC mutations are spontaneously acquired in somatic cells with overt malignancy developing only after a long period of other sequential genetic aberrations, influenced by epigenetic factors. In familial cancers, APC mutations may be passed down via germlines, and most of these adenomas are pedunculated and small. Most conventional adenomas cluster in the distal (left) colon, occurring commonly in the rectosigmoid region. Larger adenoma size is associated with an increasing prevalence of dysplasia and malignancy (Figure 6).3 Polyps <10 mm are rarely malignant and any cancerous change make take as long as 10 years. Polyps >10 mm, however, are more likely to harbor cancer, with benign polyps of that size transforming into cancer over a period of two to five years.
Figure 6:
Relationship of Conventional Adenoma Size to Likelihood to Presence of High-Grade Dysplasia
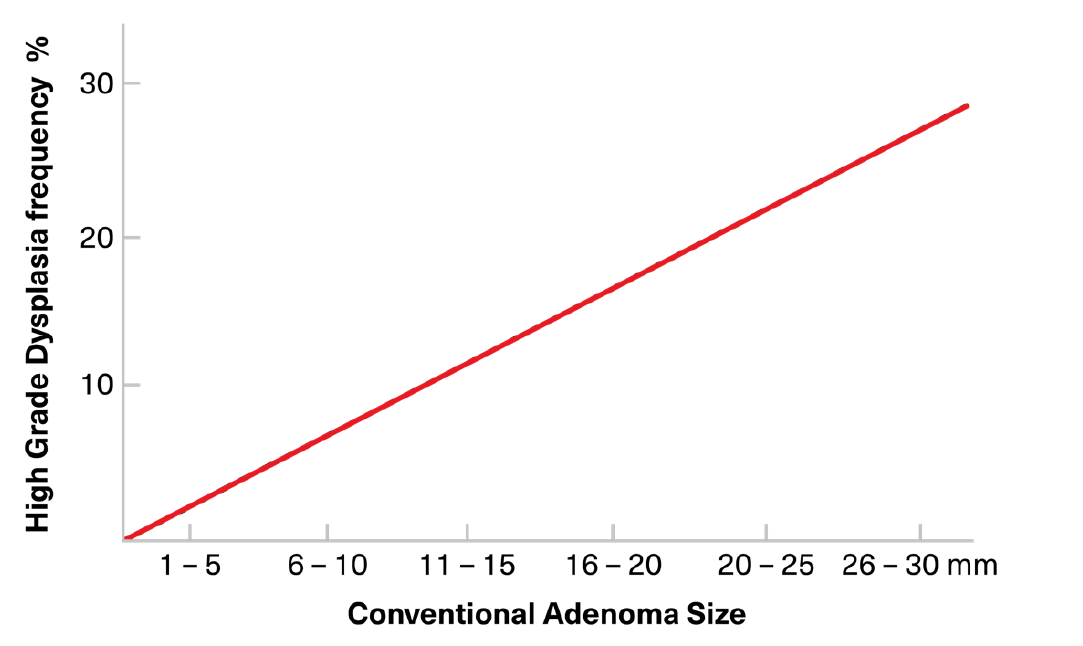
Adapted from: Markowitz AJ, Winawer SJ. Management of Colorectal Polyps. CA: A Cancer Journal for Clinicians. 1997 Mar/Apr; 47(2): 93-112.3
Conventional adenomas usually have a smooth surface, but some contain components of a villous or frondlike architecture. The two are termed “smooth conventional adenomas” and “villous conventional adenomas,” respectively. Sessile adenomas are more likely to contain villous components than pedunculated adenomas.
Conventional adenomas are subclassified by the degree to which they exhibit villous architecture. While this introduces another layer of complexity in nomenclature, it has been argued as material on the basis of malignancy potential.
Table 3: Conventional Adenoma Nomenclature |
Villous Characteristics | Type | Prevalence |
None | Tubular adenoma | 80% |
Villous architecture <80% | Tubulovillous adenoma | 10% |
Villous architecture >80% | Villous adenoma | 10% |
Traditionally, villous architecture in serrated polyps has been considered an independent predictor of cancer risk and death. Although recent data suggests that the risk may not be as high as previously thought, most surveillance guidelines maintain villous-specific protocols. The European Society of Gastrointestinal Endoscopy (ESGE), however, makes recommendations in its guidelines irrespective of villous components.
Serrated Polyps
Serrated polyps are less common than conventional adenomas and their terminology continues to evolve. The majority are sessile (flat or slightly raised with no stalk). Serrated polyps are responsible for approximately 20% of sporadic cancers although the majority of these polyps do not become cancerous. The prevalence of serrated polyps is 20%-40% in average risk individuals with the majority being hyperplastic.6
Serrated polyps tend to appear at younger ages than conventional adenomas. While prevalence steadily increases with age it does not seem to have as sharp a rise in prevalence with age as do conventional adenomas. While previously considered to represent a benign homogenous population, the recognition that this polyp category contains some entities that carry cancer-precursor mutations has resulted in the description of three main subtypes. In 2019, the WHO expanded the number of subtypes in order to better stratify the spectrum of associated malignancy risk and in recognition of the difficulties sometimes experienced at histological examination. They are:
Hyperplastic polyps (HP) These were known as metaplastic polyps in older nomenclature
Sessile serrated adenomas (SSA) or sessile serrated polyps (SSP) or sessile serrated lesions (SSL)
SSA/SSP/SSL with dysplasia
Traditional serrated adenomas (TSA) (also known as traditional serrated polyps)
HP is by far the most prevalent serrated polyp and has the lowest malignancy potential. HPs are small (<5 mm), sessile, and mostly cluster in the left colon. SSA/SSP/SSL are also sessile, but are larger than HPs (20% >10 mm) and mostly cluster in the right colon.
Large sessile lesions are subject to incomplete piecemeal endoscopic resection and early recurrence. Surveillance guidelines reflect these potential surgical difficulties.
Table 4: Serrated Polyps – Nomenclature and Characteristics |
Type | Relative Frequency | Size | Characteristics | Histology | Dysplasia | Mal-ignancy Potential |
Hyperplastic polyp (HP) | 80%-90% | <5 mm | Sessile/flat | Serrated, normal architecture | Nil; risk increased if larger size | Nil to low 20% (+) carry BRAF mutation |
Sessile serrated adenoma (SSA), sessile serrated polyp (SSP), sessile serrated lesion (SSL) | 15%-20% | <10 mm | Sessile/flat | Serrated, distorted architecture | +/- | High 80% (+) carry BRAF mutation |
Traditional serrated adenoma (TSA) | 1%-5% | >10 mm | Pedunculated | Serrated, distorted architecture | +/- | High BRAF, KRAF, or APC mutation |
The pathway to cancer in serrated polyps, known as the serrated pathway, is different from that of conventional adenomas. Mutations in the BRAF gene are common for both SSA/SSP/ SSL and TSA, and these lesions are considered to be at high risk of developing colorectal cancer. The oncogenic gene prevalence is much less common in HP. Gene expression is again influenced by epigenetic factors such as smoking, type 2 diabetes, and alcohol consumption, and dietary factors such as red meat consumption.
SSA/SSP/SSL begins to appear in the mucosa of the right colon as early as the third decade of life, becomes clinically significant around age 50, and then incidence increases rapidly from 55- 70 years of age. Increases in SSA/SSP/SSL incidence track with the age-related prevalence of DNA methylation in colonic mucosa coupled with the presence of the BRAF mutation.12 SSA/ SSP/SSL tends to grow more rapidly than conventional adenomas and are overrepresented as precursor lesions in interval colorectal cancers (CRCs diagnosed within 60 months of a negative colonoscopy).12
HPs are distinct from other serrated polyps in that they have not traditionally been seen as having the potential to transform into cancer. Although the label “adenoma” continues to be avoided, it is now recognized that HPs may harbor oncogenes and may not always be as innocuous as previously thought. Subsets of HPs may become dysplastic or transform into SSA/SSP/SSL and behave as precursor lesions. Polyps that transform have a high prevalence of the BRAF mutation.
Clinically, HPs are considered high-risk if they are multiple, large (>10 mm), or dysplastic, or if they occur in the context of a family history of serrated polyposis syndrome (SPS).
Serrated Polyposis Syndrome (SPS)
SPS has been described and is associated with excess CRC risk in the proband and in first- degree relatives, although a clear heritability pattern has not yet been established.
SPS is characterized by the presence of multiple serrated polyps. It is the most common polyposis syndrome and has a much higher prevalence than FAP. Mean age at diagnosis (45 years) is younger than that of patients with SSA/SSP/SSL (57 years) who do not meet criteria for SPS.7
Given the absence of any genetic marker, a diagnosis of SPS is defined by the WHO as follows:
- A lifetime total of >20 serrated polyps of any size (≥5 proximal to the rectum), or
- A lifetime total of ≥5 serrated lesions/polyps proximal to the rectum, all being at least 5 mm in size with at least two measuring >10 mm
An SPS diagnosis in individuals with any type of serrated polyp who have a family history of SPS had been considered a diagnostic criterion but was abandoned by WHO in 2019.
SPS carries high malignancy risk, and concurrent CRCs have been reported in as many as 15% - 30% of patients at time of diagnosis. Although as many as 70% with SPS will meet its diagnostic criteria at the first screening colonoscopy when it is discovered, it may take years and a number of colonoscopies to accumulate enough polyps for a confirmed diagnosis. This may result in under-diagnosis of SPS, although the risks associated with lack of early recognition may be mitigated by treating individual serrated polyps with care and in accordance with current accepted surveillance guidelines.
Overall risk of malignancy with SPS may be as high as 30% - 70% (compared to 100% for FAP), but the five-year risk falls to 1% once a diagnosed individual is admitted to surveillance programs. No germline genetic marker has yet been identified despite the excess risk of CRC in first- degree relatives (risk ratio=5).
Implications of Dysplasia in Histological Reports
Dysplasia describes pathological appearances reflecting abnormal cell growth characteristics that fall short of actual cancer. Dysplasia results from DNA damage and is viewed as a progression towards cancer transformation, if not an indication that transformation to cancer may have established. Although some would argue that, by definition, any neoplasm or new uncharacteristic cell growth is some degree of dysplasia, adenoma cells may have essentially normal histological morphology and may therefore not be described as dysplastic.
In pathology reports, colon lesions are usually described either as non-dysplastic or as associated with low, intermediate, or high grades of dysplasia. The degree of dysplasia in polyps is predictive of their outcomes and influences surveillance recommendations. Increasing severities of dysplasia reflect increasing aberrancy in cellular proliferation and an expression of the serial acquisition of sinister oncogenes.
Small polyps (<10 mm) contain only the earliest of genetic alterations, and only small numbers of these will develop or acquire the additional genetic changes necessary to stimulate uncontrolled accelerated cell division, marked dysplasia, and the development of cancer.
High-grade (or severe) dysplasia (HGD) now embraces the same histological characteristics as carcinoma in situ (CIS). Some authorities have abandoned the term “CIS” and instead use the term “epithelial lesions in the gastrointestinal tract,” and “HGD” has replaced “CIS” in some attending physician statements. High-grade dysplasia can only be found in <10% of conventional adenomas and sessile serrated adenomas in average risk populations, reflecting the fact that most polyps do not become cancerous.
Index Screening for Colorectal Polyps
Screening protocols for colorectal polyps vary by country and authority and continue to evolve. In low-risk individuals with no symptoms, no inflammatory bowel disease, and no family history of cancer, screening generally commences between 45 and 50 years of age.
Recommended screening modalities for average risk individuals include:
- Annual fecal occult blood (FOB) test (including high- sensitivity guaiac-based FOB tests)
- Annual fecal immunochemical test (FIT)
- Colonoscopy every 10 years
- Multitarget stool DNA test every three years
Surveillance After Polyp Resection
Updated protocols for surveillance in those with an index polyp have been published and are available online from the European Society of Gastrointestinal Endoscopy (ESGE) (2020), Cancer Council of Australia (2019), and the U.S. Multi-Society Task Force on Colorectal Cancer (2020).8, 9
In general terms, surveillance recommendations after polyp resection in average risk individuals is influenced by the following polyp aspects:
- Histology (conventional adenoma vs serrated polyp)
- Size
- Number
- Presence or absence of dysplasia
- Villous vs smooth architecture
While low-risk polyps may not require increased surveillance (return to regular screening intervals), others may require repeat colonoscopies at shorter intervals.
For males with low-risk polyps, five year intervals may be recommended if they have metabolic syndrome.
Conclusions
Colorectal cancer (CRC) is the second most common type of cancer diagnosed and the third leading cause of cancer- related mortality despite screening opportunities which are unsurpassed in oncology in terms of proven effectiveness.
As screening becomes more frequently accessed and adopted, insurers need to recognize the very high prevalence of polyps and understand their malignancy potentials. While careful screening with follow-up surveillance can reduce CRC incidence and mortality, as colonoscopies are being undertaken at increasing rates, pressure is increasing on medical services.
Screening guidelines continue to emphasize the importance of high-quality examinations, with resections being undertaken and patients followed up with care and rigor. Reassurance that a desired outcome will be achieved following resection of polyps detected during screenings requires an understanding of polyp classification and behavior and a grasp of the procedures involved.
Finally, risk assessment of individuals with high-risk precancerous lesions must be undertaken with reference to the pathology of index lesions discovered and ensuring that surveillance protocols, appropriately tailored to the individual’s circumstances, are in place and followed.
The author would like to thank Dr. Radhika Counsell, Consulting Medical Officer, RGA, for her peer review of this article.



 Adapted from: Malki A, et al. Molecular Mechanism of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2021; 22(1): 130.18
Adapted from: Malki A, et al. Molecular Mechanism of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2021; 22(1): 130.18


