For more than 20 years, medical science has seen an explosion of productive research into neuroinflammation (i.e., inflammatory response affecting the nervous system).
Researchers have also studied neuroinflammation's impact on many adverse health conditions including neurodegenerative disorders such as dementias and Parkinson’s disease, an array of psychiatric conditions, and persistent pain states.
The new knowledge sets aside long-held but apparently incorrect beliefs about the structure and function of the nervous system and related biological processes in the human body.
Inflammation has long been recognized as a process triggered by tissue injury or assault, with the primary purpose of defending against and repairing after the injury or assault. It involves an array of actions that include specific immunological functions of a number of cell types, each with a different set of functions. Some types will ingest and remove substances which threaten the individual experiencing the inflammation. Others will release chemical mediators, of which there are many different types with distinct but sometimes overlapping roles.
Inflammation’s processes are integral to the body’s survival. They are triggered by infection or injury and are of prominent relevance in conditions such as heart disease, cancer, diabetes, and arthritis. The role of inflammation in both peripheral and central sensitization in pain states has long been recognized. It has also been recognized that, with varying modes, durations, and circumstances, inflammatory activity and its expressions are not always beneficial and indeed may become deleterious, as typified by, for example, rheumatological disease.
Glial Cells and Neuroinflammation
Among the long-held beliefs now being set aside are those related to the glial cells of the central nervous system (CNS). The collective noun for this group of cells originated in the Greek word for “glue” and was applied to a range of CNS cells previously considered essentially inactive, with its only role that of providing scaffolding for the neurons, the active nerve cells of the nervous system. One type of glial cell, the oligodendrocyte, was recognized as providing the very important myelin sheaths around the axons of fast acting nerve cells, but that was all.
Over the last few decades, an understanding has emerged that glial cells do in fact perform a range of the important functions relevant to neuroinflammation within the CNS (see Table 1).
Table 1: Types of Glial Cells, Their Functions, and Implications |
Cell Type | Role in Inflammation | Implications in Pain |
Microglia | Respond to pathogens/injury – migrate as required Removal of damaged cells/ debris Release cytokines, chemokines, prostaglandins, reactive oxygen species, ATP
| Stimulated by repeated exposure to opioids, causing release of inflammatory mediators which amplify pain and distort pain signaling |
Astrocytes | Regulate transmitter release from neurons Regulate blood-brain barrier Promote oligodendrocyte activity Moderate new neuron formation (neuroplasticity)
| Involved in response to neuronal stress or injury; can promote nerve destruction as well as nerve repair / rebuilding |
Oligodendrocytes | | Activity in spinal cord implicated in genesis and maintenance of chronic pain states |
Ependymal cells | | Not known as yet |
The inflammatory response is not always the same wherever it occurs. It will vary according to several factors, including the nature and site of the injury or assault, its duration, and elements specific to the individual. Neuroinflammation, for example, can have positive or negative impacts. On the one hand, it can be associated with the removal of dangerous elements and reorganization of a repairing nervous system, but on the other, it can be associated with additional damage which can manifest in many ways. Further, neuroplasticity, an important process in recovery from a CNS injury, has been shown to be either enhanced or reduced, depending on different types of neuroinflammatory activity.
Notably, while elevated inflammatory activity has been detected in the CNS in instances of physical adversity, it is also found when there is emotional threat, and has been shown to be associated with adverse mood states such as anxiety. This biological association of mood/anxiety with the inflammation found in conditions such as persistent pain may be an important insight for patients and the practitioners caring for and treating them.2
Further to the inflammatory activity of the glial cells themselves, the mediating chemicals they release cause recruitment of inflammatory response cells from the bloodstream. The activity of the latter is facilitated by altered permeability of the blood-brain barrier. That barrier comprises the endothelial cells which line all blood vessels as well as, in the context of the CNS, projections of the astrocytes, the most abundant of the glial cells. It is the astrocytes which are primarily responsible for the altered permeability of the barrier.
Glial cells do in fact perform a range of the important functions relevant to neuroinflammation within the CNS.
Microglia, a type of glial cell, have two roles – primary immune surveillance and ingestion and removal of unwelcome cells. Comprising 10% to 15% of the cells in the CNS, microglia provide important neuroprotective roles, and as their turnover is slow, they are less susceptible to long-term damage. When confronted by disease and varying with the type of injury or assault, microglia produce inflammatory chemicals which promote the recruitment of other protective cells. Unfortunately, in certain situations, microglial activity can become excessive and/or persistent to the detriment of the individual. Such enhanced activity has been implicated in conditions such as persistent pain states, neurodegenerative conditions, and mental illnesses.3
The Glia’s Connections
Enmeshed with the advancing knowledge of neuroinflammation is the role of dietary factors. Gut microbiota, the term applied to the microorganisms which colonize the gastrointestinal tract, are known to alter according to a number of variables, not least of which are a person’s dietary habits.
Gut microbiota are now increasingly recognized as asserting influence outside of the gastrointestinal tract. This includes the CNS, with established links to cognitive function and immune responses. Research has linked microglial dysfunction, causing chronic neuroinflammation and neurological disorder, to gut microbiome imbalance.4 The microbiome has also been found to impact hypothalamic-pituitary- adrenal (HPA) activity, which is implicated in the development of neurological and psychiatric disorders as well as of fatigue syndromes.
A bidirectional role for communication between brain and gut is also postulated for the vagus nerve, and numerous studies point to its influence on microglial neuroinflammatory activity.
Proinflammatory chemicals are also known to be released by the excessive fat stores carried by overweight individuals, which can increase pain perception and complaint.
Finally, and importantly, there is emerging research on the role of a healthy liver in all of these aspects of pain and its management, which appears to be impacting these systems. The liver- brain axis is recognized as influencing such processes as oxidative stress in the CNS, and other adverse metabolic processes involved with neurodegeneration and central sensitization of pain processing.7
The Links to Pain
In relation to pain specifically, there is increasing recognition that two types of glial cells, astrocytes5 and microglia, have significant roles in the emergence and persistence of amplified pain states, derangement of neuro-organization (as occurs in windup or central pain sensitization), and the reduced efficacy of opiate medications. The activity of these cells involves the release of several inflammatory chemicals as well as modulating chemicals and growth factors, which can lead to derangement of healthy neural structures and networks within the spinal cord and brain. Although these cell types assert little (if any) action in normal pain situations, these cells are involved in changes which cause amplification of pain through these inflammatory actions. Unfortunately, their activation is augmented by persistent opioid use, and the glia themselves demonstrate further increases in activity in response to chronic opiate exposure. The impact is amplification of pain, the spread of pain beyond its original distribution, changes in the type and intensity of pain, and reduced effectiveness of opioid analgesics.
Figure 1:
Aspects of Neuroinflammation
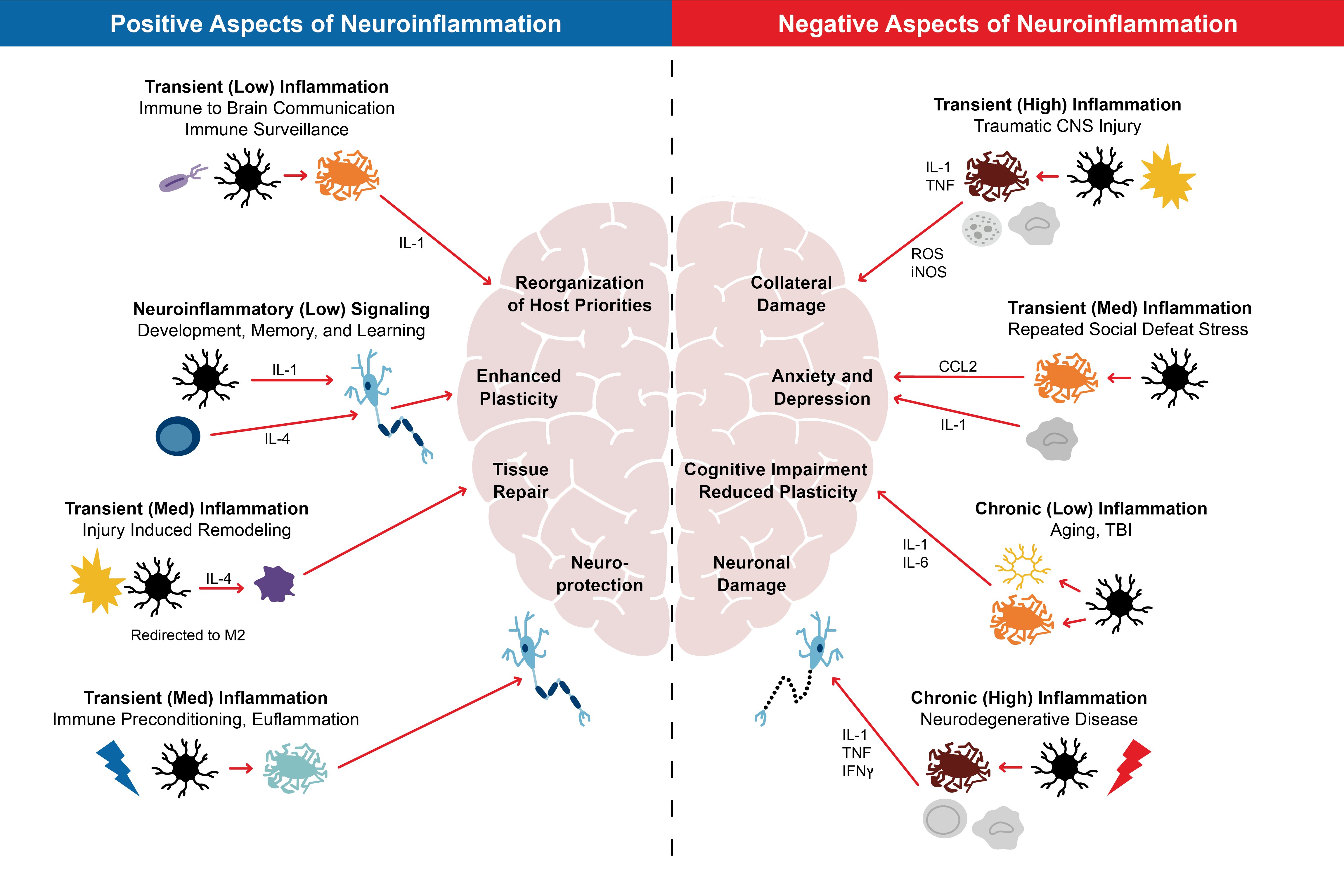
Source: Adapted from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5025335/figure/F1/
Implications for Treatment
The most important conclusion to be drawn from the emerging research is that persistent pain states are not usually a disorder of the identified affected body part. While there may be ongoing pathology of the organ or body part, there will also be derangement within the nervous system, particularly in the spinal cord and brain. It is for this reason that persistent pain should perhaps be considered a disease or condition in its own right, beyond the identified affected body part.6
There are many driving factors that contribute to persistent pain, and all must be recognized and addressed.
Attempts to treat pain with strategies that focus solely on the initial injury or disease are failing to acknowledge and address the nervous system pathology which can ensue, and which can augment and perpetuate the patient’s distress and dysfunction.
Research continues to delve into biological approaches to manipulating the mechanisms cited in this article. Some are less specific, such as stimulation of the vagus nerve, while others are more focused attempts to manipulate the activity of centrally acting inflammatory chemicals released by the glia and other implicated cells. This has stimulated interest in the possibility of harnessing these mechanisms with anesthetic and even antibiotic agents, but as yet no solid therapeutic advances have been made.
The message to patients with persistent pain and their medical caregivers is that there are many driving factors which contribute to persistent pain, and all must be recognized and addressed. As indicated, these include an undue emphasis on treating the presumed source of pain, the ongoing use of opiate medications, and the failure to address psychological factors associated with stress. Ideally, this emerging research should be explained to the patient with a view to promoting insight into persistent pain as a disease state of both the whole nervous system and the whole person. That insight should also be used to encourage clinical adoption of a “whole person” approach to improving the body and brain’s proneness to inflammation while reversing the drift to overall deterioration.


 Source: Adapted from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5025335/figure/F1/
Source: Adapted from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5025335/figure/F1/