Early detection of disease in apparently healthy individuals has an intuitive appeal arising from the notion that early diagnosis is synonymous with cure.
Screening originated as a medical strategy for the purposes of identifying occult disease in individuals before the development of symptoms and signs. The enthusiasm with which screening was adopted was based on the idea that the deployment of treatment strategies at a preclinical stage would limit advanced disease and reduce mortality.
With time, the scope of screening has broadened to include the identification of those at an increased risk of disease and has come to encompass the collection of genetic data for that purpose.
Screening is no longer confined to the field of medical practice, where it is undertaken selectively in accordance with an individual’s clinical risk profile and where there are proven opportunities to improve health outcomes. Screening is now undertaken outside of clinical settings by various service providers and potentially improperly promoted. Too often, testing is driven by poorly informed consumer demands and incorrectly targeted without due regard for the predictive value and the consequences of the findings.
Too often, testing is driven by poorly informed consumer demands and incorrectly targeted without due regard for the predictive value and the consequences of the findings.
The data gathered, even when collected with informed consent, may not be owned by the individual and may be used by providers in ways that are not open to the consumer or capable of scrutiny. The utility of screen-detected findings, particularly if deployed indiscriminately outside of evidence-based mainstream settings, needs to be interpreted by trained professionals with an understanding of the performance characteristics of the test within the environment in which it was applied.
The mere availability of screening techniques does not imply that testing should be broadly applied without a considered focus, although the current environment is such that this is increasingly the case.
Screening outcomes become powerful determinants of costly medical interventions and are important drivers of decisions taken outside of clinical medicine on behalf of people not seeking medical help.
Inappropriate screening may harm healthy individuals, squander resources, trigger extensive reflex testing and result in unintended outcomes. While ever more sensitive screening modalities generate important data regarding the true burden of disease, the ramifications are not necessarily advantageous.
The mere availability of screening techniques does not imply that testing should be broadly applied without a considered focus.
Genomic screening, particularly when undertaken in a non-targeted fashion without a clearly defined intent, has introduced a new layer of extreme complexity to the screening environment. The interpretation and application of broad-based genetic data requires considerable expertise, which includes an understanding of gene expression and penetrance. It needs to be recognized that there are many influences which come to bear on the development of a phenotype, or the manifestation of disease, in those who might be genetically predisposed.
From an insurance perspective, screening test results need to be managed carefully with due consideration of the context in which the test was undertaken and with a clear understanding of the degree to which the results can be validly applied to the individual being assessed.
Identifying conditions that are known to be highly prevalent in the applicant population, and that are already accommodated in premium structures, can lead to prejudicial decisions that are overly conservative.
Findings that identify a risk for some future health event need to be interpreted and managed with respect to the degree to which any lag time might exist before there is any insurance impact.
Identifying conditions that are known to be highly prevalent in the applicant population, and that are already accommodated in premium structures, can lead to prejudicial decisions that are overly conservative.
This paper will focus on early disease detection, and not on predictive testing or genetic screening, although the application of genetic information for insurance purposes, where permitted by law, is coming to the fore and increasingly the province of those with specialized training and expertise.
Screening for Disease
Screening for disease refers to the application of a test in populations with no signs or symptoms of disease with the aim of detecting the targeted condition and treating that condition before it becomes clinically evident.
These tests were developed for clinical purposes and are not diagnostic in themselves. They are investigations that identify individuals who require further evaluation in order to rule in (confirm) or rule out (exclude) the condition in question.
Despite the notion that early detection provides an opportunity for curative intervention, it cannot be assumed that all those with a screen-detected abnormality will benefit from that early diagnosis.
Medically accepted screening programs are governed by ethical imperatives that require evidence that the benefits outweigh any harm and proof that outcomes are advantageous and not disadvantageous to the individual. Indiscriminate screening, driven by consumer demand, nonmedical forces and commercial interests, may not always see those boundaries respected.
Despite the notion that early detection provides an opportunity for curative intervention, it cannot be assumed that all those with a screen-detected abnormality will benefit from that early diagnosis.
Understanding Screening Test Performance
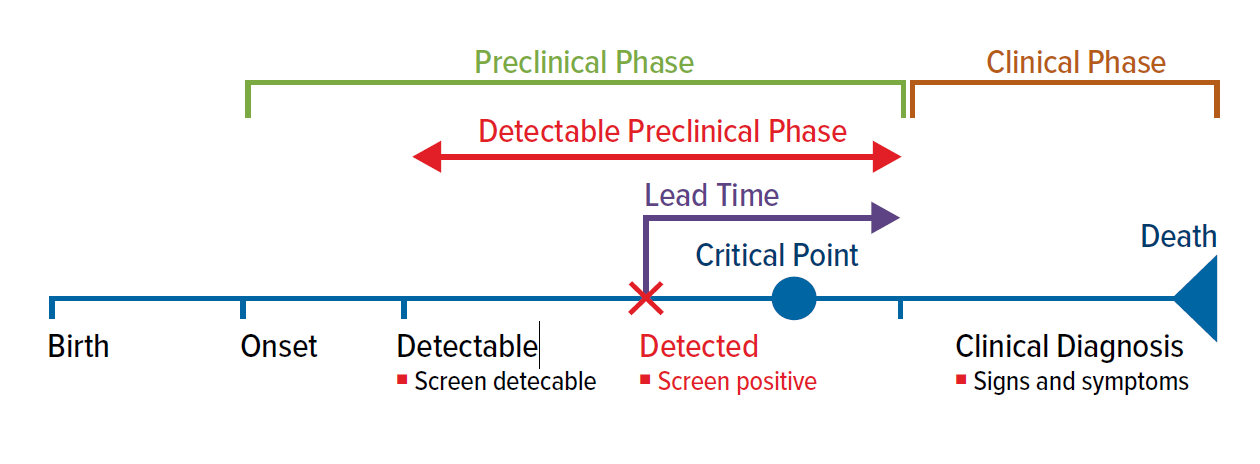
Screening can only be justified if a test, applied during the preclinical phase of a disease, has the potential to positively influence the outcome.
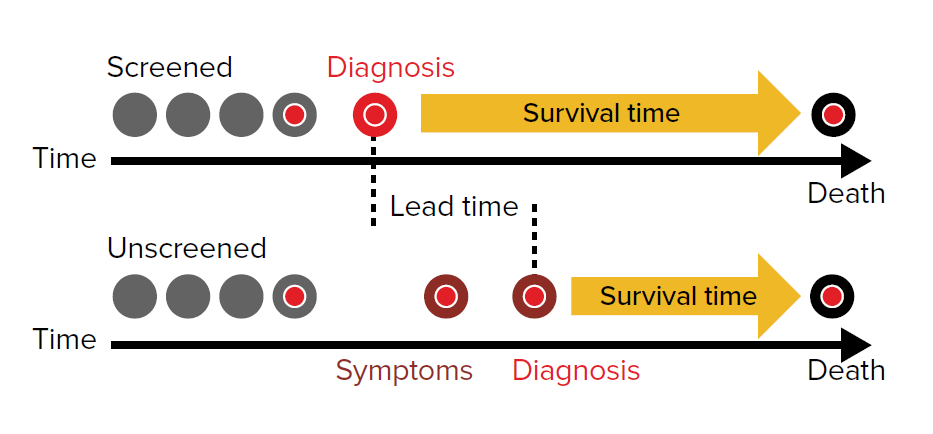
The time from a screen-detected diagnosis to the time a clinical diagnosis would otherwise have been made is called the lead time. The test becomes useful when a preclinical diagnosis is able to be made before a critical therapeutic point is reached. The critical therapeutic point is the moment after which treatments do not influence the outcome of a screen-detected condition (Fig. 1).
Survival improvements that appear to be a consequence of a screening diagnosis can be more illusory than real and must be interpreted in the context of disease behavior. An apparent survival advantage after a screen detected diagnosis should not necessarily be interpreted as proof that an early diagnosis leads to a benefit.
Figure 1
An ideal screening test would accurately and reliably discriminate between those with, and those without, a condition.
A perfect test would have the capacity to identify the condition in all those with that condition and would miss no cases. All those with the condition would test positive and there would be no false negatives. That test would be deemed to have a sensitivity of 100%.
Also, a perfect test would have the potential to correctly identify all those who do not have the condition and not detect any abnormality in healthy individuals. All those without the condition would test negative and there would be no false positives. Such a test would be deemed to have a specificity of 100%.
Unfortunately, no screening test has perfect precision.
Imperfect sensitivity results in conditions being missed (false negatives) and imperfect specificity results in individuals without the condition being incorrectly labelled as having that condition (false positives).
The pursuit of full sensitivity, driven by the quest to miss no cases, is usually associated with a loss of specificity that exposes some individuals to unnecessary investigations and the associated hazards and costs.
The pursuit of full sensitivity, driven by the quest to miss no cases, is usually associated with a loss of specificity that exposes some individuals to unnecessary investigations.
When determining the value and applicability of any particular screening modality consideration must be given to the degree to which there is any tradeoff between sensitivity and specificity.
What is considered acceptable will depend on the implications of missing a crucial diagnosis when judged against the implications of mislabeling and overdiagnosis.
While increasing test sensitivity has resulted in the capacity to identify the true prevalence of disease it has resulted in the diagnosis of some conditions that might never have led to any adverse health consequence during the insured’s lifetime. This is referred to as over-diagnosis and leads to unnecessary treatment and anxiety.
Both incorrect labelling and over-diagnosis have important insurance consequences with increased exposure to lump sum living benefits and health costs.
From an insurance perspective the interpretation of a screening test result ultimately comes down to its predictive value.
The predictive value of a test expresses the degree to which a result can be relied upon to have correctly determined the status of the individual.
The positive predictive value (PPV) is the degree to which a positive test can be relied upon to have correctly labelled an individual with a condition and the negative predictive value (NPV) is the degree to which a negative test has correctly labelled an individual without the condition. It is expressed as a percentage and is calculated from the tests capacity to result in true and false positives and true and false negatives.
No conclusion can be drawn on the implications of results without knowing the probability of the condition being present in the population being tested.
No conclusion can be drawn on the implications of results without knowing the probability of the condition being present in the population being tested. This notion is expressed in the Bayesian probability theory which states that the probability of a test result providing valid information requires a consideration of how likely any given person is to have a disease in the first place (the pretest probability) and the sensitivity and specificity of the screening tool.
If the prevalence of a disease in a population or an individual is low, it is inevitable that there will be more false positive results than true positive results and the test will have little predictive value. The predictive value of a positive test approaches zero if the population screened is essentially free of the condition in question.
A positive test result is likely to be a true positive (correctly identifying the presence of the disease) if deployed in populations or individuals with a high chance of having the disorder. A positive test result is likely to be a false positive if deployed in an individual with a very low chance of having the condition.
Similarly, the validity of negative tests must be questioned if they do not fit the predetermined pretest likelihood of disease.
While screening for certain conditions in appropriately targeted populations carries a recognized advantage there are many considerations which need to be examined when assessing the value of protocols. Proof of effectiveness of a screening tool is best confirmed by a demonstrable effect on mortality. Other measures of effectiveness are often quoted and have pitfalls which have the potential to introduce bias.
Understanding Bias
There are a number of biases that need to be understood when assessing the merits of screening protocols; these include lead time bias, length time bias, and over-diagnosis bias.
- Lead time bias
Lead time bias refers to diagnoses which result in an improved survival time following a diagnosis while not influencing the actual time of death. Although the period of time to death differs by mode of diagnosis (screened vs. unscreened), the time of death may not have been influenced (Fig. 2).
Lead time bias creates a false impression that an early diagnosis has produced a benefit, whereas screening might only have resulted in a longer survival time after diagnosis than might otherwise have been the case.
- Length time bias
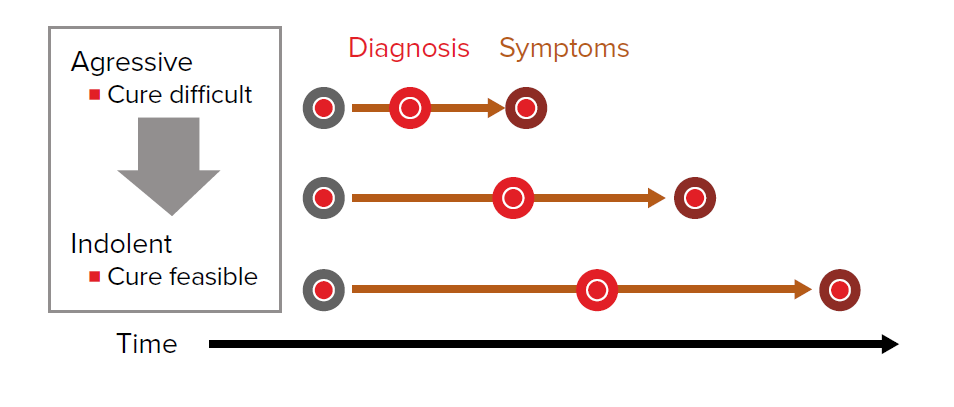
Length time bias refers to the fact that the probability of screen detection is related to the speed of disease development and progression.
Very fast-growing cancers have a short window between the potential for detection by screening and the development of symptoms. These cancers may develop and progress rapidly to a prognostically disadvantageous stage between traditional screening intervals. In these situations screening may be deemed to be ineffective at identifying early disease and in modifying the prognosis.
Slow-growing indolent cancers of the same organ are more likely to be screen-detectable because the asymptomatic phase is protracted. Screening will be deemed to have produced a benefit by way of early diagnosis and the apparent value enhanced by that fact that the tumor, by its very nature, is easily cured (Fig. 3).
Persons with slowly progressive disease will be overrepresented in cohorts and their survival will be naturally longer. The longer survival may be wrongly attributed to early detection and therapy.
- Over-diagnosis bias
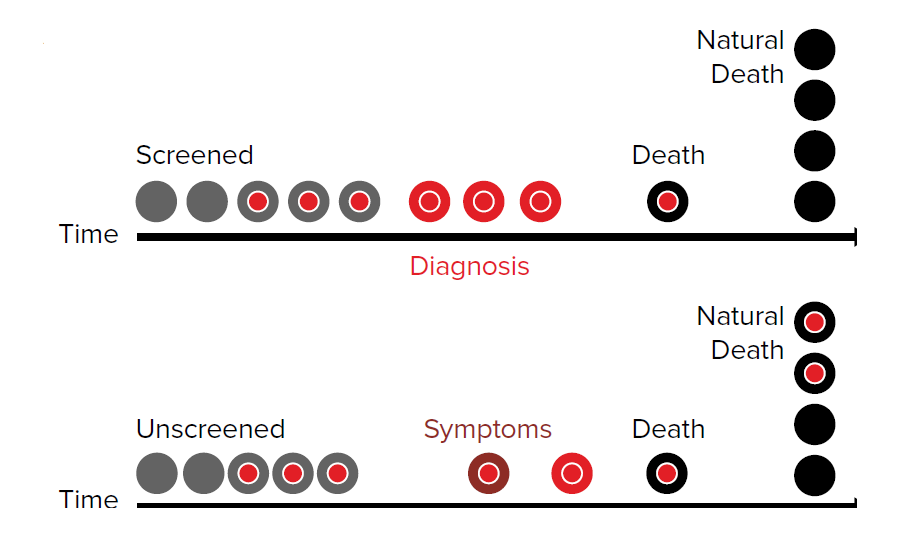
Over-diagnosis bias is an extreme of length time bias.
This refers to the screening diagnosis of indolent disease that would not have caused excess mortality had it never been diagnosed in the first place (Fig. 4).
Treatment results in an apparent survival improvement in the screened population and can be improperly interpreted as evidential support for testing. Outcomes should be adjusted for over-diagnosis bias before any screening program is deemed useful.
Over-diagnosis, or the identification of pseudo-disease, leads to unwarranted anxieties and adverse treatment related outcomes. Over-diagnosis and over treatment increases critical illness and disability exposure and may actually increase all-cause mortality
Population screening takeawaysPopulation screening for disease is only defensible where:1. The disease is prevalent. 2. The disease has serious consequences. 3. The preclinical phase is long enough to permit a reasonable testing frequency. 4. There is a capacity to detect disease before the critical therapeutic point is reached. 5. The test has a high detection potential (good sensitivity). 6. Testing detects little irrelevant disease (good specificity). 7. The over-diagnosis rate and the consequences of over-diagnosis are acceptable. 8. The test carries an acceptable risk without significant morbidity. 9. Effective treatment for the disease is available. 10. The treatment is more effective if administered in the preclinical phase. |
Bias or Benefit
While the protective value and health benefits of properly directed screening programs are not in question, over-diagnosis and overtreatment have become an unavoidable consequence of ever more sensitive screening modalities.
The adverse outcomes of screening healthy individuals have become increasingly important and the cost, stigma, and potential injury associated with screening outcomes must not be ignored in the quest for earlier diagnoses. This has become particularly evident in the over-diagnosis associated with screening for cancer.
The adverse outcomes of screening healthy individuals have become increasingly important and the cost, stigma, and potential injury associated with screening outcomes must not be ignored in the quest for earlier diagnoses.
Mitigation of the potential adverse outcomes of screening and the avoidance of mislabeling and overtreatment will likely come in the guise of an increased understanding of disease behavior and an enhanced capacity to abandon the “one size fits all” therapeutic approach that currently follows many early diagnoses.
A better understanding of diagnostic outcomes and changes in disease labelling, driven by genetic determinants and other disease mediators, will be central to solving that concern.
Applying Screening in the Insured Population
While benefits for screening certainly exist, a proper understanding of the pitfalls and challenges of disease screening is crucial to the application of screening protocols in insurance medicine.
Screening in one form or another will remain part of risk selection if anti-selection is to be avoided and if the cross subsidization of substandard applicants by healthy populations is to be minimized.
Individuals who see themselves as healthy and risk averse might reasonably expect assessments to take their individual circumstances into account with a view to maximizing the benefits of healthy lifestyle choices and minimizing premium imposes.
An increasing focus on individual risk stratification has many implications, including pricing imperatives within otherwise broadly aggregated pools.
Maximizing the utility of screening for insurance purposes will require the industry to select and deploy meaningful tools which have an acceptable degree of precision and which are not considered to be overly intrusive by applicants. It will also require the industry to acquire a knowledge base that allows the results of screening tests, undertaken outside of the underwriting process and disclosed at application, to be applied accurately and with fairness.
There will inevitably be a need to carefully consider trade-offs.
Generally speaking, screening will continue to focus on predictive modelling at the younger ages (where even high risk parameters have had no time to be expressed as a phenotype) but perhaps move more to a disease prevalence screening model at the older ages if we are to assess applicants more as individuals.
Screening for phenotypic expression has the potential to modify traditional risk-factor based underwriting but integrating multiparametric models requires a considerable understanding of disease behavior and a determination of how these models interact and integrate.